New USF Health research on endothelial-derived microvesicles, using models of sepsis, may be useful for better diagnosis and treatment of inflammatory or infectious diseases
A new preclinical study by the University of South Florida Health (USF Health) Morsani College of Medicine sheds light on how tiny bubble-like particles flowing in the blood can serve as diagnostic markers for certain diseases while also contributing to disease progression.
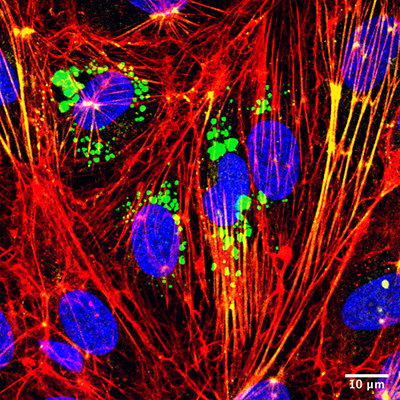
Microvesicles (green) interacting with target endothelial cells (nuclei stained blue) to increase both stress fiber formation (red) and activation of cellular contraction proteins (yellow). The interaction contributes to greater vascular wall barrier permeability (leakage). Image courtesy of Victor Chatterjee (University of South Florida), originally published by Oxford University Press, Cardiovascular Research, https://doi.org/10.1093/cvr/cvz238
Cells lining the inner surface of blood vessels, called endothelial cells, have the ability to package and release microscopic vesicles (0.1 to 1 micrometer in diameter) into the blood circulation. These microvesicles carry unique cargo of molecules under different health or disease conditions; thus, by identifying their specific cargo content or molecular signature, doctors can better diagnose the nature and extent of a medical problem.
Researchers in the laboratory of Sarah Yuan, MD, PhD, at the USF Health Department of Molecular Pharmacology and Physiology, discovered that endothelial cells produce microvesicles containing a high level of c-Src protein during sepsis, a life-threatening condition that causes systemic inflammation and multiple organ failure. Their study, conducted using cell cultures and an animal model of sepsis, was recently reported in Cardiovascular Research, a highly rated journal sponsored by the European Society of Cardiology.
Most intriguingly, the researchers found that in addition to providing a unique marker that signifies the status of inflammation in blood vessels, these c-Src enriched microvesicles play an active role in causing vascular wall injury and barrier leakage.
Victor Chatterjee, MD, PhD, a postdoctoral fellow in the Department of Molecular Pharmacology and Physiology, was the paper’s first author. He works in the laboratory of departmental chair Sarah Yuan, MD, PhD. | Photo by Allison Long, USF Health Communications and Marketing
“Microvesicles produced by inflamed endothelial cells circulate in the blood and target healthy barrier cells by unloading their bioactive cargo into receiving cells. They ‘tell’ the receiving cells to change behavior, leading to an increased permeability of the barrier,” said first author Victor Chatterjee, MD, PhD, a postdoctoral fellow working in Dr. Yuan’s lab.
Like the breech of a protective levy, increased permeability of the endothelial barrier allows blood fluids and proteins to leak through the blood vessel wall into surrounding tissues. Because this leak process underlies sepsis, traumatic injury, atherosclerosis, cancer, and several types of inflammatory or immunological disorders, the authors suggest that endothelial-derived microvesicles may have potential applications in developing new molecular markers or therapeutic targets for better diagnosis and treatment of these diseases.
This paper also reports an in-depth analysis of the molecular mechanisms underlying vascular leakage caused by endothelial derived microvesicles.
A key finding is that the circulating microparticles are highly interactive. They bind to the membrane of targeted endothelial cells and get inside these cells, where they unload c-Src cargo to turn on the signal for cell contraction and cell-to-cell junction opening. Since junctions are critical structures that “glue” neighboring cells together to form the vascular wall barrier, opening them results in blood leakage.

Dr. Yuan, a member of the USF Health Heart Institute, was senior author of the NIH-supported study published in Cardiovascular Research.
In an effort to translate their benchwork to bedside care, the USF Health researchers plan to use blood samples from human patients to determine if and how the molecular signature of microvesicles change over time or correlate with disease severity, Dr. Chatterjee said. A better understanding of how these tiny cargo carriers function in the human disease process could help guide physicians in better managing infectious or inflammatory diseases, he said.
The senior author of the Cardiovascular Research paper is Dr. Yuan, professor and department chair, who holds the USF Health Deriso Endowed Chair in Cardiovascular Disease. Dr. Yuan’s research has been supported by the National Institutes of Health: National Heart, Lung, and Blood Institute, and National Institute of General Medical Sciences.
