USF Health researchers applied CRISPR technology to study the very large human non-protein coding gene expressed only in placenta, stem cells and certain cancers
TAMPA, Fla (March 16, 2020) — The placenta, an organ which attaches to the lining of the uterus during pregnancy, supplies maternal oxygen and nutrients to the growing fetus. Abnormal formation and growth of the placenta is considered an underlying cause of various pregnancy complications such as miscarriages, stillbirth, preeclampsia and fetal growth restriction. Yet, much remains to be learned about molecular mechanisms regulating development of this blood-vessel rich organ so vital to the health of a pregnant woman and her developing fetus.

Hana Totary-Jain, PhD, an associate professor of molecular pharmacology and physiology in the USF Health Morsani College of Medicine, was senior author of the study published in Scientific Reports.
University of South Florida Health (USF Health) Morsani College of Medicine researchers recently discovered how a very large human non-protein coding gene regulates epithelial-to-mesenchymal transition (EMT) – a process that contributes to placental development during early pregnancy, but can also promote cancer progression.
During the first trimester, fetal-derived placental cells known as trophoblasts invade the maternal uterine lining and modify its blood vessels to allow oxygenated blood to flow from the mother to fetus. However, trophoblast invasion requires tight regulation of EMT. If inadequate, trophoblast invasion is too shallow to adequately remodel the maternal blood vessels, and adverse pregnancy outcomes can occur. Conversely, excess EMT can cause exaggerated trophoblast invasion through the uterine wall leading to placenta accreta, a condition that can cause hemorrhage and often requires hysterectomy at delivery.
The USF Health researchers used a powerful genome editing technology called CRISPR (shorthand for “CRISPR-dCas9) to activate all of the chromosome 19 microRNA cluster (known as C19MC), so they could study the gene’s function in early pregnancy. C19MC — one of the largest microRNA gene clusters in the human genome — is normally turned off but becomes expressed only in the placenta, embryonic stem cells and certain cancers.

Dr. Totary-Jain discusses the molecular aspects of placenta development and pregnancy complications with research collaborator Umit Kayisli, PhD, a professor of obstetrics and gynecology at USF Health.
In their cell model study, published Feb. 20 in Scientific Reports, a Nature research journal, the USF Health team showed that robust activation of C19MC inhibited EMT gene expression, which has been shown to reduce trophoblast invasion.
But when trophoblast-like cells were exposed to hypoxia – a lack of oxygen similar to that occurring in early placental development — C19MC expression was significantly reduced, the researchers found. The loss of C19MC function causes differentiation of trophoblasts from stem-like epithelial cells into mesenchymal-like cells that can migrate and invade much like metastatic tumors. This EMT process helps explain trophoblast invasion and early placental formation.
“We were the first to use CRISPR to efficiently activate the entire gene, not just a few regions of this huge gene, in human cell lines,” said the paper’s senior author Hana Totary-Jain, PhD, an associate professor in the Department of Molecular Pharmacology and Physiology, USF Health Morsani College of Medicine. “Our study indicates C19MC plays a key role in regulating many genes important in early implantation and placental development and function. The regulation of these genes is critical for proper fetal growth.”

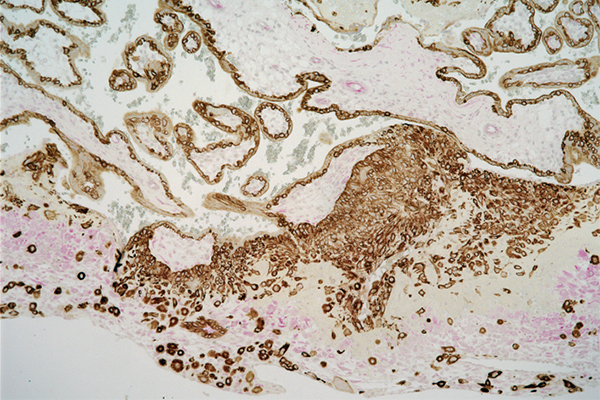
Above: Chromosome 19 microRNA cluster (stained purple) expressed in first-trimester placenta. Below: In preparation for pregnancy, fetal trophoblast cells (brown) from which the placenta arises invade maternal decidual cells (pink) in the uterus lining. | Images courtesy of Hana Totary-Jain, originally published in Scientific Reports: doi.org/10.1038/s41598-020-59812-8
“You need EMT, but at some point the process needs to cease to prevent adverse pregnancy outcomes,” Dr. Totary-Jain said. “You really need a balance between not enough invasion and too much invasion, and C19MC is important in maintaining that balance.”
Dr. Totary-Jain and others in her department collaborated with colleagues in the medical college’s Department of Obstetrics and Gynecology on the project.
“The USF Health study offers new insight into how trophoblasts interact with the maternal uterine environment to become more invasive or less invasive in the formation of the placenta,” said coauthor Umit Kayisli, PhD, a USF Health professor of Obstetrics and Gynecology. “More research on microRNA expression and how it inhibits EMT may help us better understand the causes and potential prevention of preeclampsia and fetal growth restriction, which account for 5-to-10 percent of all pregnancy complications as well as spontaneous preterm births.”
Investigating the effects of altered C19MC expression on cell differentiation and trophoblast invasion has implications not only for a better understanding of normal and abnormal placental development, but also for cancer and stem cell research, Dr. Totary-Jain added.
Photos by Freddie Coleman, USF Health Communications and Marketing