Mice deficient in a receptor needed to safely clear unresolved cardiac inflammation may help in discovering therapies for heart failure with preserved ejection fraction
TAMPA, Fla (June 22, 2020) — A receptor that plays an essential role in safely clearing chronic unresolved cardiac inflammation may offer new targets for treating an increasing type of heart failure associated with age-related obesity, suggests a preclinical study led by researchers at the University of South Florida Health (USF Health) Heart Institute, Morsani College of Medicine.
Heart failure with preserved ejection fraction, or HFpEF, is one of two types of heart failure – both characterized by shortness of breath, exercise intolerance, fatigue and fluid retention. Unlike the second type of heart failure, known as heart failure with reduced ejection fraction, HFpEF currently has no FDA-approved drugs to improve the weakened heart’s pumping function.

Ganesh Halade, PhD, USF Health associate professor of cardiovascular sciences, was senior author of the The FASEB Journal paper on age-related obesity and heart failure with preserved ejection fraction.
More than half of all patients with clinical heart failure have HFpEF, a growing public health problem because of the aging population and growing incidence of obesity. In HFpEF, the heart contracts normally and seems to pump out a normal proportion of blood; however, the heart muscle can thicken and weaken causing the ventricle to withhold an abnormally small volume of blood. So, while the heart’s output as measured by ejection fraction may appear within the normal range, it is insufficient to meet the body’s demands.
In a study published June 16 in The FASEB Journal, the USF Health-led team identified a mouse model that thoroughly mimics HFpEF syndrome in humans. These obesity-prone mice lack the inflammation clearing (resolution) receptor, ALX/FPR2 or ALX for short — a deficiency previously shown to drive cardiac and kidney inflammation in aging mice.
Using this unique model, the researchers defined how the ALX resolution receptor promotes the activity of specialized proresolving mediators (SPMs), fatty-acid derived signaling molecules. These SPM molecules support the body’s innate immune response to help clear out chronic inflammation and advance cardiac repair following an acute heart attack. Conversely, the researchers noted that sustained, unresolved inflammation after heart attack can aggravate abnormalities in endothelial cells lining the heart and kidneys. These abnormalities prompt endothelial dysfunction that changes blood vessel integrity — a primary sign of both age-related obesity and HFpEF.

Dr. Halade (center) with his research team, postdoctoral fellow Bochra Tourki, PhD, (left) and research associate Vasundhara Kain, PhD, (right), in their USF Health Heart Institute laboratory.
“Remarkably, the deficiency of a single receptor triggers obesity in mice at an early age and this, in turn, gives rise to many molecular and cellular processes ultimately leading to heart failure with preserved ejection fraction,” said senior author Ganesh Halade, PhD, associate professor of cardiovascular sciences at the USF Health Heart Institute.
The FASEB study’s three key findings were:
- The obesity-prone ALX-deficient mice had increased food intake and impaired energy metabolism compared to normal mice (with a working ALX receptor) of the same ages. The obesity-driven metabolic dysfunction led to heart structural remodeling, defective cardiac electrical activity and weakened heart muscle.
- Deletion of the ALX receptor increased ion channel gene expression and disrupted multiple ion channels, which supported electrocardiogram evidence of heart rhythm disturbances in the mice.
- Obesity-prone, ALX-deficient mice develop heart muscle damage characteristic of HFpEF with steady inflammation in the heart and kidneys. This suboptimal inflammation is directed remotely by immune cells (leukocytes) in the spleen and advanced by dysfunctional (leaky) cardio-renal endothelial tissue in older ALX-deficient obese mice.
Overall, the research describes the importance of the resolution receptor essential for SPM action, particularly resolvins that suppress the inflammatory response to acute injury without compromising a healthy immune response. In fact, a specific resolvin (D1) is a key that unlocks the ALX resolution receptor to enable pharmacological action and, eventually, safe clearance of inflammation, Dr. Halade said.
The study offers insight into potential targeted treatments for HFpEF that would harness the benefits of naturally-produced SPMs. Omega 3-rich diets and/or SPM supplements to preserve the receptor’s normal function may help prevent this type of heart failure, Dr. Halade said, while SPMs or other molecules specifically designed to reactivate a dysfunctional receptor might help treat existing HFpEF.
This latest research builds upon previous work by Dr. Halade’s laboratory – all focused on discovering the best ways to prevent, delay or treat the unresolved inflammation influencing heart failure. The team’s goal is to contribute to individualized therapies that may account for possible sex, racial/ethnic or age-related physiological differences.

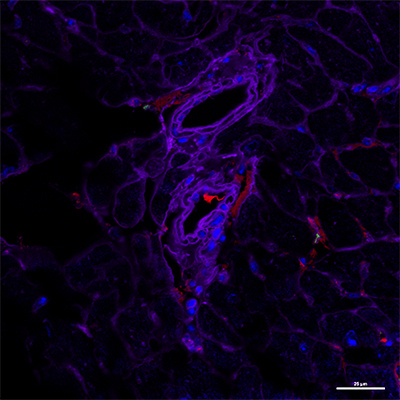
Absence of the inflammation resolution receptor ALX triggers heart muscle endothelial dysfunction: Immunofluorescent microscopic image (below) shows a decrease in expression of CD31 (endothelial cells stained red) in a 4-month old, obesity-prone mouse deficient in the ALX receptor, compared to same-age normal mouse with a functioning receptor (above). Images courtesy of Halade laboratory; appeared first in The FASEB Journal, Jun 16, 2020, doi: 10.1096/fj.202000495RR
Approximately, 6.5 million Americans have heart failure, which contributes to one in every eight deaths, according the Centers for Disease Control and Prevention.
The USF Health researchers collaborated with Charles N. Serhan, PhD, DSc, Harvard Medical School; Saame Raza Shaikh, PhD, University of North Carolina at Chapel Hill; and Xavier Leroy, Domain Therapeutics, France. The team science study was supported in part by grants from the NIH’s National Heart, Blood and Lung Institute.
– Photos by Allison Long, USF Health Communications and Marketing