]]>
In search of individualized heart failure therapies, Ganesh Halade leads a USF Health Heart Institute team studying unresolved inflammation after heart attack
//www.youtube.com/watch?v=KarBPXZs1rs
Short-term inflammation is one of the body’s key defense mechanisms to help repair injury and fight infection. But low-level inflammation that does not subside has been linked to many common chronic conditions, including cardiovascular diseases such as atherosclerosis, atrial fibrillation and heart failure.
Ganesh Halade, PhD, an associate professor of cardiovascular sciences at the USF Health Morsani College of Medicine, investigates the safe clearance of acute inflammation – and what happens at the molecular and cellular levels when initially beneficial inflammation becomes harmful to the heart.  His team at the USF Health Heart Institute works on bridging the gap between the immune-responsive metabolism of fat and cardiac health by more clearly defining two distinct but simultaneous processes: the inflammatory response and how inflammation is safely cleared, or resolved.
In particular, Dr. Halade’s laboratory focuses on discovering ways to prevent, delay or treat the unresolved inflammation after a heart attack, which plays a key role in the pathology leading to heart failure. Their goal is to contribute to individualized therapies that may account for possible sex, racial/ethnic or age-related physiological differences in heart failure, a leading cause of hospitalizations and deaths worldwide.

Ganesh Halade, PhD, associate professor of cardiovascular sciences, joined the USF Health Heart Institute in February 2020. [Photo by Allison Long, USF Health Communications]
“Although several treatments and devices exist to help manage heart failure, the challenge remains the growth of metabolic risk factors like obesity, diabetes, hypertension and aging that amplify heart failure – and inflammation underlies all these conditions,†Dr. Halade said. “We’re in the early stages of understanding how the inflammatory response becomes chronic, or unresolved†after heart attack-induced injury.
Honing in on “the rootsâ€
Dr. Halade’s late father, a farmer in Nashik close to Mumbai, India, emphasized to his young son that if he wanted to make a difference in life to “look to the roots, rather than the fruits.â€
That philosophy drives Dr. Halade’s research endeavors. “We focus on the root causes of inflammation so that we can successfully treat the chronic inflammation that leads to heart failure,†he said.

Dr. Halade (center) with his research team, postdoctoral fellow Bochra Tourki, PhD, (left) and research associate Vasundhara Kain, PhD, (right). [Allison Long, USF Health Communications]
Clinical trials of several anti-inflammatory therapies so far have failed to show benefit in heart failure patients. Dr. Halade suggests that the investigational compounds intended to suppress inflammation very early in the cardiovascular disease process likely disrupt the tight control of immune-responsive signaling needed for timely resolution of inflammation.
“The inflammatory response and its resolution are two sides of the same coin – and they roll together. Blocking one side will affect the other,†he explained. “So, we don’t want to block the ‘get in signal’ needed to promote the early, ‘good’ inflammation. We want to accelerate the ‘get out’ signal to immune cells, so that as soon as repair of cardiac injury is done the acute inflammation leaves without becoming chronic.â€

Dr. Halade views a high-resolution image (below) of a normally beating heart. [Photos by Anne DeLotto Baier, USFH Research Communications]
Connecting dysfunctional inflammation control and heart failure
A class of immune-system molecules orchestrates the resolution of tissue inflammation, an active process essential for advancing cardiac healing after a heart attack. These specialized proresolving mediators, or SPMs, are signaling molecules that form when fatty acids metabolize in response to immune activation of leukocytes.
Dr. Halade’s work is helping uncover new details on how heart failure-inducing inflammation may be limited (without promoting immunosuppression) – either by administering pharmacological SPMs, or activating enzymes that help stimulate the body’s own SPMs.
Over the last two years, he has published significant findings in several leading journals (papers summarized below) making the connections between fatty acids, inflammation control, and heart failure. Among Dr. Halade’s study collaborators is Charles Serhan, PhD, of Harvard Medical School, a pioneer in the emerging field of inflammation resolution.
- Science Signaling: This study followed the time course of inflammation and its resolution in a mouse heart attack model. The research showed for the first time that the active inflammation-resolving phase coincided with the acute inflammatory response facilitating cardiac repair after a heart attack. Among other factors, the researchers looked at types and amounts of SPMs, and the expression of enzymes that synthesize SPMs, both in the spleen and at the injured site of the heart. Macrophages, a type of white blood cell, are needed to generate SPMs as opposed to other immune cells, they reported.

Dr. Halade’s laboratory focuses on discovering ways to prevent, delay or treat the unresolved inflammation after a heart attack, which plays a key role in the pathology leading to heart failure. [Anne DeLotto Baier]
- Journal of the American Heart Association: The preclinical study discovered male-female cardiac repair differences in heart failure survival after heart attack, including improved recovery of cardiac function and greater survival of acute and chronic heart failure in female mice. Females generated higher levels of a particular fatty acid-derived signaling molecule (EET; epoxyeicosatrienoic acids) known to facilitate healing after a heart attack.
- ESC Heart Failure: The researchers profiled bioactive lipids (inflammatory biomarkers) in blood samples from hospitalized Black and White patients soon after a severe heart attack. They found a potent SPM signature (resolvin E1) was significantly lower in Black men and women than in Whites. The study concluded bioactive lipids are key for the diagnosis and treatment of cardiac repair after heart attack to delay heart failure.
- The FASEB Journal: Halade and colleagues identified a mouse model to study heart failure with preserved ejection fraction (HFpeF), a common form of heart failure linked to age-related obesity. Using this unique model of obese aging, they defined how the deficiency of a single resolution receptor triggers obesity in mice at an early age, which can give rise to many of the molecular and cellular processes ultimately leading to HFpEF.

Vasundhara Kain (seated) and Bochra Tourki, look at slides for a paper on age-related obesity and heart failure. [Allison Long, USF Health Communications]
Insight into potential inflammation-resolving therapies
As they learn more about the metabolic and immune-responsive signals that control acute cardiac inflammation, researchers hope to harness the capacity of fatty acid-derived bioactive molecules to prevent, diagnose and treat heart failure, Dr. Halade said. SPMs are derived primarily from omega-3 fats in our diet – the polyunsaturated “good†fats in foods like salmon, avocados, almonds, and walnuts.
Some evidence indicates that omega 3-rich diets and/or SPM supplements, as well as getting enough exercise and quality sleep may help prevent the unresolved inflammation leading to heart failure, Dr. Halade said. If SPMs are not produced due to risk factors like obesity or aging, or because enzymes required to metabolize fatty acids are deficient, then drugs specifically designed to facilitate cardiac repair and calm inflammation might delay or treat heart failure, he added. Distinctive biochemical signatures acquired by analyzing SPMs or other metabolites might even be used to help diagnose heart failure or predict which treatments will work best for certain patients.
Dr. Halade joined USF Health this February from the University of Alabama at Birmingham, where he was a faculty member since 2013. He received his PhD in pharmacology from the University of Mumbai Institute of Chemical Technology in 2007. He completed two postdoctoral fellowships at the University of Texas Health Science Center in San Antonio. The first fellowship focused on nutritional immunology. The second was conducted with mentor Merry Lindsey, PhD, to examine the effects of obesity on post-heart attack cardiac structure and function.

Foods rich in omega-3 fatty acids (including salmon, walnuts and avocados), as well as enough exercise and quality sleep, may help prevent unresolved inflammation contributing to cardiovascular disease.
Dr. Halade’s research is supported by funding from the NIH’s National Heart, Lung and Blood Institute. In 2018, he received American Physiological Society Research Career Enhancement Award to train in lipidomics at the RIKEN Center for Integrative Medical Sciences in Japan.
His inflammation resolution research has been recognized with two awards for studies published in the American Journal of Physiology-Heart and Circulatory. An Article Impact Award 2020 was conferred this March by the American Physiological Society for Dr. Halade’s work defining the impact of the cancer drug doxorubicin on the heart and spleen. He also received a 2017 Best Paper Award from the Unbound Science Foundation. Dr. Halade is associate editor for the American Journal of Physiology-Heart and Circulatory and for Scientific Reports, and serves on the editorial boards of several other high-impact journals in cardiovascular sciences.

At left: Beneficial resolution of inflammation following cardiac repair. At right: Risk factors like aging, obesity and some medications can contribute to unresolved (chronic) inflammation, which impairs cardiac repair and can lead to heart failure. [Graphic courtesy of Ganesh Halade]
Some things you may not know about Dr. Halade
- As an undergraduate student in India, Dr. Halade won the gold medal in fencing at a statewide collegiate competition.
- To help promote a heart healthy lifestyle, he enjoys recreational bicycling and gardening in his backyard, where he grows vegetables and chiles.
- Halade lives in Tampa with his wife Dipti, an information technology engineer, and their son Arav, 13.

Top:Â Sources of inflammation include injury (like damage from a heart attack), infection (viruses, bacteria or other pathogens), and factors associated with lifestyle (such as poor diet and lack of exercise). Below: Ways to help prevent unresolved cardiac inflammation associated with lifestyle. [Graphics courtesy of Ganesh Halade]
-Video by Allison Long, USF Health Communications and Marketing
]]>
]]>
Mice deficient in a receptor needed to safely clear unresolved cardiac inflammation may help in discovering therapies for heart failure with preserved ejection fraction
TAMPA, Fla (June 22, 2020) — A receptor that plays an essential role in safely clearing chronic unresolved cardiac inflammation may offer new targets for treating an increasing type of heart failure associated with age-related obesity, suggests a preclinical study led by researchers at the University of South Florida Health (USF Health) Heart Institute, Morsani College of Medicine.
Heart failure with preserved ejection fraction, or HFpEF, is one of two types of heart failure – both characterized by shortness of breath, exercise intolerance, fatigue and fluid retention. Unlike the second type of heart failure, known as heart failure with reduced ejection fraction, HFpEF currently has no FDA-approved drugs to improve the weakened heart’s pumping function.

Ganesh Halade, PhD, USF Health associate professor of cardiovascular sciences, was senior author of the The FASEB Journal paper on age-related obesity and heart failure with preserved ejection fraction.
More than half of all patients with clinical heart failure have HFpEF, a growing public health problem because of the aging population and growing incidence of obesity. In HFpEF, the heart contracts normally and seems to pump out a normal proportion of blood; however, the heart muscle can thicken and weaken causing the ventricle to withhold an abnormally small volume of blood. So, while the heart’s output as measured by ejection fraction may appear within the normal range, it is insufficient to meet the body’s demands.
In a study published June 16 in The FASEB Journal, the USF Health-led team identified a mouse model that thoroughly mimics HFpEF syndrome in humans. These obesity-prone mice lack the inflammation clearing (resolution) receptor, ALX/FPR2 or ALX for short — a deficiency previously shown to drive cardiac and kidney inflammation in aging mice.
Using this unique model, the researchers defined how the ALX resolution receptor promotes the activity of specialized proresolving mediators (SPMs), fatty-acid derived signaling molecules. These SPM molecules support the body’s innate immune response to help clear out chronic inflammation and advance cardiac repair following an acute heart attack. Conversely, the researchers noted that sustained, unresolved inflammation after heart attack can aggravate abnormalities in endothelial cells lining the heart and kidneys. These abnormalities prompt endothelial dysfunction that changes blood vessel integrity — a primary sign of both age-related obesity and HFpEF.

Dr. Halade (center) with his research team, postdoctoral fellow Bochra Tourki, PhD, (left) and research associate Vasundhara Kain, PhD, (right), in their USF Health Heart Institute laboratory.
“Remarkably, the deficiency of a single receptor triggers obesity in mice at an early age and this, in turn, gives rise to many molecular and cellular processes ultimately leading to heart failure with preserved ejection fraction,†said senior author Ganesh Halade, PhD, associate professor of cardiovascular sciences at the USF Health Heart Institute.
The FASEB study’s three key findings were:
- The obesity-prone ALX-deficient mice had increased food intake and impaired energy metabolism compared to normal mice (with a working ALX receptor) of the same ages. The obesity-driven metabolic dysfunction led to heart structural remodeling, defective cardiac electrical activity and weakened heart muscle.
- Deletion of the ALX receptor increased ion channel gene expression and disrupted multiple ion channels, which supported electrocardiogram evidence of heart rhythm disturbances in the mice.
- Obesity-prone, ALX-deficient mice develop heart muscle damage characteristic of HFpEF with steady inflammation in the heart and kidneys. This suboptimal inflammation is directed remotely by immune cells (leukocytes) in the spleen and advanced by dysfunctional (leaky) cardio-renal endothelial tissue in older ALX-deficient obese mice.
Overall, the research describes the importance of the resolution receptor essential for SPM action, particularly resolvins that suppress the inflammatory response to acute injury without compromising a healthy immune response. In fact, a specific resolvin (D1) is a key that unlocks the ALX resolution receptor to enable pharmacological action and, eventually, safe clearance of inflammation, Dr. Halade said.
The study offers insight into potential targeted treatments for HFpEF that would harness the benefits of naturally-produced SPMs. Omega 3-rich diets and/or SPM supplements to preserve the receptor’s normal function may help prevent this type of heart failure, Dr. Halade said, while SPMs or other molecules specifically designed to reactivate a dysfunctional receptor might help treat existing HFpEF.
This latest research builds upon previous work by Dr. Halade’s laboratory – all focused on discovering the best ways to prevent, delay or treat the unresolved inflammation influencing heart failure. The team’s goal is to contribute to individualized therapies that may account for possible sex, racial/ethnic or age-related physiological differences.

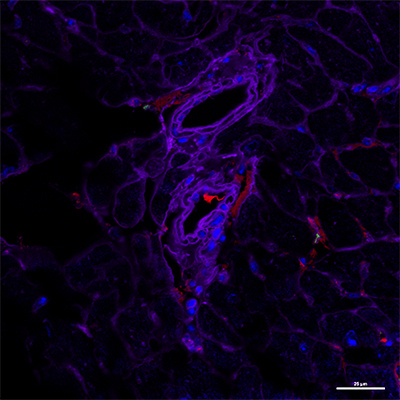
Absence of the inflammation resolution receptor ALX triggers heart muscle endothelial dysfunction: Immunofluorescent microscopic image (below) shows a decrease in expression of CD31 (endothelial cells stained red) in a 4-month old, obesity-prone mouse deficient in the ALX receptor, compared to same-age normal mouse with a functioning receptor (above). Images courtesy of Halade laboratory; appeared first in The FASEB Journal, Jun 16, 2020, doi: 10.1096/fj.202000495RR
Approximately, 6.5 million Americans have heart failure, which contributes to one in every eight deaths, according the Centers for Disease Control and Prevention.
The USF Health researchers collaborated with Charles N. Serhan, PhD, DSc, Harvard Medical School; Saame Raza Shaikh, PhD, University of North Carolina at Chapel Hill; and Xavier Leroy, Domain Therapeutics, France. The team science study was supported in part by grants from the NIH’s National Heart, Blood and Lung Institute.
– Photos by Allison Long, USF Health Communications and Marketing
]]>