]]>
Researchers at the USF Health Morsani College of Medicine were awarded $5.6 million of expected funds for a 4-year study from the U.S. Department of Defense to examine why many people with Friedreich’s Ataxia (FA) go on to also develop heart disease, a major cause of death for those with FA.
Principal investigator for the USF study is Thomas McDonald, MD, professor in the Department of Internal Medicine (Division of Cardiology) and the Department of Molecular Pharmacology and Physiology in the USF Health Morsani College of Medicine. Dr. McDonald is also a researcher in the USF Health Heart Institute and director of the USF Health Cardiogenetics Clinic.
“We still don’t have a full understanding of the genetic mutation for Friedrich’s ataxia to determine why so many patients go on to get heart disease – we need to know,†Dr. McDonald said. “The physiology is not well characterized. This study will help us gain a better understanding of the basic mechanisms of the gene that carries FA, and help identify clinical predictors of the FA-associated heart disease.â€
The new study dovetails with current work taking place in Dr. McDonald’s lab, including an R56 grant from the National Institutes of Health, which focuses on the fundamental mechanisms of LMNA-associated heart disease passed from one generation to the next — and what can be done to help prevent disease and its consequences.
This FA-heart disease study will follow FA patients and their parents over four years, and will involve careful clinical monitoring of heart health, examination of biomarkers, whole genome sequencing, stem cell modeling of heart tissue, and mitochondrial function studies.

From left, Dr. Kami Kim, Dr. Aarti Patel, Dr. Thomas McDonald, and Dr. Theresa Zesiewicz. Not pictured is Sami Noujaim, PhD.
Spearheading the work in the DoD study is a multidisciplinary team of USF Health experts representing cardiology, genetics, neurology, molecular pharmacology, cardiac electrophysiology and predictive modeling. The diverse expertise will help distinguish the clinical, genetic, and biological factors that contribute to cardiac disease in FA patients. Data from FA families and basic science models will be integrated with clinical data to identify unique factors in the heart that influence the cardiac phenotype and separate cardiac-specific traits from those influencing the neurological phenotype.
“Study results could lead to tools used in patient care settings to identify those FA families most at risk for cardiomyopathy and allow for potential intervention and treatment that could help delay onset of the heart disease,†Dr. McDonald said.
The USF Health interdisciplinary team for the study includes:
- Thomas McDonald, MD: clinical cardiology, molecular pharmacology and cardiogenetics (Division of Cardiology, Department of Internal Medicine, MCOM)
- Aarti Patel, MD: neurocardiogenetics and cardiac imaging (Division of Cardiology, Department of Internal Medicine, MCOM)
- Sami Noujaim, PhD: molecular pharmacology and cardiac electrophysiology (Department of Molecular Pharmacology and Physiology, MCOM)
- Kami Kim, MD: machine learning and clinical predictive modeling (Division of Infectious Diseases, Department of Internal Medicine, MCOM; Center for Global Health Infectious Diseases Research, COPH)
- Theresa Zesiewicz, MD, clinical neurology (Department of Neurology, MCOM)
Dr. Zesiewicz, professor in MCOM and director of the USF Health Ataxia Research Center, has specialized in clinical research and patient care for ataxias and other movement disorders’ for more than 20 years and is recognized as an international expert and leader in the field of hereditary ataxias. Her movement disorders clinic supports the evaluation of over 3,000 patients per year, likely the busiest in the world.
“Dr. Zesiewicz will play a vital role in recruiting research participant and in overseeing neurological assessments of patients as they are longitudinally followed in this study,†Dr. McDonald said.
The funding for the study came from the DoD through its Congressional Directed Medical Research Programs (CDMRP), a section of DoD that funds novel approaches to biomedical research. Link: https://cdmrp.health.mil/
The team will begin recruiting study participants next month.
Photo by Ryan Rossy, USF Health Communications
]]>
]]>
//www.youtube.com/watch?v=xAWQcqqrnIs
USF Health has established a Neurocardiogenetics Clinic focused on improving the quality of life for patients with hereditary neuromuscular disorders who are at risk for or already experiencing cardiac complications. Innovative genetic research is a central component of the new clinic.
The multidisciplinary clinic teams faculty and staff with clinical expertise in cardiology, genetics and neurology. The clinic is directed by Aarti Patel, MD, assistant professor of cardiology and director of the Cardiac Imaging Fellowship Program at USF Health in collaboration with Thomas McDonald, MD, professor of cardiology and molecular pharmacology and physiology, and a member of the USF Health Heart Institute, and Theresa Zesiewicz, MD, professor of neurology and director of the USF Health Ataxia Research Center. Patients receive a complete cardiac examination and testing along with a comprehensive family genetic evaluation from providers familiar with their neurological history.
“Combining all that information, we are able to come up with a personalized treatment plan,†Dr. Patel said.
Many patients initially seen at the Neurocardiogenetics Clinic have been diagnosed with Friedreich’s ataxia (FA), a rare, debilitating and life-shortening neuromuscular disorder that usually strikes in childhood. At some point in their lives 55 to 60-percent of FA patients develop some form of cardiac disease. The most devastating of these is cardiomyopathy, a heart muscle disease that limits blood pumped to the rest of the body and increases risk for heart failure and abnormal heart rhythms.
“FA is a multisystem disease that affects many different parts of the body, including the heart,†Dr. Zesiewicz said. “And one of its most sinister complications is cardiomyopathy, an enlarged heart.â€
The Neurocardiogenetic clinic offers patients and their families the opportunity to participate in ongoing genetic studies exploring potential links between heart disease and neuromuscular diseases like FA and certain types of other ataxias and muscular dystrophies.

Dr. Aarti Patel (left), assistant professor of cardiology, collaborates with Dr. Thomas McDonald, professor of molecular pharmacology and physiology, at the new USF Health Neurocardiogenetics Clinic. Dr. Theresa Zesiewicz. professor of neurology (not pictured), is another collaborator.
While a deficiency in the frataxin protein can lead to fibrosis and scarring of heart muscle tissue in FA, competing theories exist about how a genetic mutation may actually result in heart problems in this particular ataxia and other neuromuscular disorders.
Dr. McDonald’s research team is searching for answers to many questions, including how different genetic variations may lead to cardiac abnormalities and affect the severity of heart disease.
Small blood samples are collected from patient volunteers with different inherited neuromuscular and/or cardiac disorders, as well as family members who are unaffected genetic carriers of neuromuscular diseases. In laboratory cell culture dishes, USF Health researchers genetically reprogram the blood cells into pluripotent stem cells that can grow into any cell type. Then they induce these stem cells — containing the same genetic make-up as the patient who provided blood — to become nerve cells and heart muscle cells.
The goal is to work out at a molecular level how FA or other inherited neuromuscular diseases damage the heart muscle. Once that is achieved, Dr. McDonald said, the “disease in a dish†can be used to identify potential drug targets and test treatment options on the patient’s own cells.
“With stem cell technology and disease-in-a-dish modeling, we’re looking for the earliest changes in either a heart or a nerve cell (that lead to disease),†he said. “A big unanswered question is whether the molecular or cellular process happening in the nerve is the same as that damaging the heart muscle. By using stem cell-based research we hope to be able to determine if there is a connection between the nerve and the heart – or whether it’s a separate, independent process but based upon the same genetic defect.â€
The collaborative research builds upon robust data collected by doctors in the clinic, including the wide range of symptoms seen in patients with hereditary neurological disorders involving cardiac complications.
Ultimately, that can help improve understanding of why genetic variations in certain neuromuscular diseases cause cardiac complications, Dr. McDonald said. “Having a clear clinical picture of each research participant will help us correlate the genetics and function of cells we see in the laboratory with how that translates to disease progression in the individual.â€
“With careful clinical surveillance and novel biomarkers, our hope is that we will be able to predict which patients and their family members are at highest risk for cardiovascular disease so we can intervene early with effective treatment,†Dr. Patel said. “We’re hoping to identify patients even before they develop cardiac symptoms, so we can prevent heart disease.â€
-Video and photos by Allison Long, USF Health Communications and Marketing
]]>
]]>
Hosted by FARA and the USF Ataxia Research Center, the annual scientific symposium emphasizes a patient-centered approach to research
//www.youtube.com/watch?v=XZtgZuXQlGQ
For the first time in its seven-year history, the Friedreich’s Ataxia Scientific Symposium brought together several pharmaceutical industry leaders to discuss preclinical and clinical studies. The companies are all attacking Friedreich’s ataxia (FA) on different fronts with the same goal in mind: to get the first treatment for the rare, but devastating, neuromuscular disease approved and on the market as soon as possible.
The Sept. 17 symposium, hosted by the Friedreich’s Ataxia Research Alliance (FARA) and the USF Ataxia Research Center, drew an audience totaling more than 500, both live at the USF Marshall Center Ballroom and viewing the event in real-time through Ustream’s CureFA channel.

The symposium featured a discussion by leading representatives of pharmaceutical and biotechnology companies working with FARA and academia to conduct new research attacking Friedreich’s ataxia on several fronts.
Representatives and supporters of FARA, the research community, and patient and their families – many whom attended FARA’s Energy Ball gala on Saturday evening, Sept. 19 — were welcomed by USF President Judy Genshaft.
The translational center of excellence at USF is “one of the most active in the world, testing potential new drugs for Friedreich’s ataxia,†President Genshaft said. “We are unstoppable in the fight for a cure for FA.â€
In the last seven years, the staff of the USF Ataxia Research Center, focused on identifying and developing effective treatments for inherited ataxia disorders, has expanded to eight, including two clinicians, a fellow, nurses and research coordinators. Center Director Theresa Zesiewicz, MD, professor of neurology, gave an overview of the center’s eight clinical trials — six active and two finishing up. USF, one of 10 sites in the international FARA Collaborative Clinical Research Network, is recruiting patients for two of the three trials presented by pharmaceutical industry leaders at the symposium. The USF center is also working with Agilis, one of the biopharmaceutical companies at the symposium, to develop their gene therapy protocol, expected to be submitted in 2016 to the Food and Drug Administration (FDA).

USF President Judy Genshaft welcomed attendees to the USF Tampa campus for the seventh annual scientific symposium “Understanding Energy for A Cure,” hosted by the Friedreich’s Ataxia Research Alliance and the USF Ataxia Research Center.
Friedreich’s ataxia is triggered by a single genetic defect that limits production of frataxin, a protein vital to the function of the energy-producing factories, or mitochondria, of the cell. This leads to a variety of symptoms including neurodegeneration that can cause muscle weakness and loss of coordination and balance, energy deprivation and fatigue, vision impairment, slurred speech, aggressive scoliosis, diabetes and life-shortening cardiac disease. Most young people diagnosed with Friedreich’s ataxia require a cane, walker or wheelchair by their teens or early 20s. There are currently no approved treatments.
FARA President Ron Bartek and Executive Director Jennifer Farmer spoke about the progress in Friedreich’s ataxia research worldwide and the value of the organization’s 2,600-member patient registry in bringing together all its stakeholders.

FARA’s Executive Director Jennifer Farmer and President Ron Bartek.
Featured speaker Sanjay Bidichandani, MBBS, PhD, the chair of pediatric medical genetics at the University of Oklahoma College of Medicine and member of the FARA Board of Directors, was part of the team that first identified the Friedreich’s ataxia gene in 1996.  Fueled by resources and partnerships cultivated by FARA, Dr. Bidichandani said, the understanding of the disease process advanced relatively quickly since that genetic discovery and has yielded robust expansion of investigational drugs in the treatment pipeline.
A panel moderated by Dr. Bidichandani featured representatives from four biotechnology and pharmaceutical companies conducting Friedreich’s ataxia research in collaboration with FARA and academia – Jeffrey Sherman, MD, executive vice president for research and development and chief medical officer, Horizon Pharma; Jodi Cook, PhD, vice president of operations, Agilis Biotherapeutics; Colin Myer, MD, chief medical officer, Reata Pharmaceuticals; and Robert De Jager, MD, chief medical officer, Retrotope. Their newly activated studies cover a range of therapeutic targets, including finding ways to boost frataxin production, improving mitochondrial function, reducing mitochondrial damage and oxidative stress, and delivering gene therapy.

Featured speaker Dr. Sanjay Bidichandani, a member of FARA’s Board of Directors, was part of the group that discovered the gene for Friedreich’s ataxia in 1996. He gave an overview of the tremendous progress in less than 20 years leading to a robust treatment pipeline.
“FARA has really galvanized the patient community, academia, industry and even regulators to develop better insights into this disease and how we can all work together,†said Horizon Pharma’s Dr. Sherman. “At the end of the day, they’ve really brought to the forefront the importance of patient centricity and the voice of the patient in clinical and basic science research.â€
Horizon Pharma has repurposed a drug already approved by the U.S. Food and Drug Administration for use in treating two other rare genetic disorders, chronic granulomatous disease and severe malignant osteopetrosis. The company recently launched a Phase 3 randomized, double-blind, placebo-controlled trial to test the safety and effectiveness of Actimmune® (interferon gamma-1b) in improving neurological function in 90 Friedreich’s patients at four U.S. sites. Previous research indicated that Actimmune®, which mimics a protein made by the body to help prevent infection, increases frataxin levels to reduce nerve cell depletion and muscle atrophy.

The symposium brought together representatives and supporters of FARA and the research community with patients in their families.
Agilis focuses on engineering and delivering therapeutic DNA to replace the damaged frataxin gene. Preclinical studies are employing a safe virus to optimally deliver the corrective gene to key targets, allowing safe and effective long-term expression of the frataxin protein.
Earlier this year Reata began a Phase 2 randomized, placebo-controlled, double-blind trial testing the safety and effectiveness of various dose levels of the oral medication RTA 408 in treating Friedreich’s ataxia. Known as the MOXle study, the trial is enrolling patients at sites worldwide, including USF. Preclinical studies have shown that RTA 408 directly activates antioxidative pathways to improve mitochondrial function.
This month USF enrolled the first of 18 patients in Retrotope’s 28-day randomized, double-blind controlled trial evaluating the safety of the investigational oral drug RT001 in ambulatory patients with Friedreich’s ataxia. USF and the University of California Los Angeles will be the only two sites for the Phase 1 study. The compound RT001 is a stabilized fatty acid shown to shut down the toxic free radical degradation of polyunsaturated fats, an essential component of cell membranes, and reduces further damage to the mitochondria.
The scientific panel was followed by a question-and-answer session on patients’ perspectives of living with Friedreich’s ataxia. The panel was moderated by symposium host Clifton Gooch, MD, professor and chair of the Department of Neurology at the USF Health Morsani College of Medicine.
The four patient participants, all diagnosed in their teens or early 20s, emphasized a common theme – that they choose every day to carry on and live life to its fullest despite the challenges of Friedreich’s ataxia.
Jade Perry, 25, was accompanied to the stage by the service dog she trained, a labradoodle named Bo.  Perry, who is finishing up a master’s degree in education from Coastal Carolina University, recently got her first full-time teaching job.
“I love riding my trike and try to keep my schedule packed so FA can’t slow me down,†she said.

Kendall Harvey, center, was diagnosed with later-stage Friedreich’s ataxia three years ago at age 25. “In spite of my diagnosis, my family and I are living life to the fullest,” she said.
Kendall Harvey, 28, diagnosed at age 25 with later-onset Friedreich’s ataxia, still walks unassisted. She and her husband live in Austin, TX, with their 11-month old son Brooks.
Harvey, who participated in volleyball, track and other sports as a youth, said she noticed problems with agility and balance while taking dance lessons in preparation for her 2013 wedding. She chalked it up to getting a little older or “being out of shape.â€Â But continuing symptoms finally led her to a neurologist who ran a battery of tests, culminating with a full genetic panel.
“The first time I heard of FA was the day I was diagnosed,†Harvey said. “FA has changed my perspective. Now, I live more in the moment than worrying about all the things in the future.
“On the days when I stumble a little more, my speech is slurred and I’m angry that my body is not behaving the way I’d like, my son is a fantastic reminder that I’m still capable of amazing things. He’s very humbling.â€

In his closing remarks Dr. Clifton Gooch, professor and chair of neurology at the USF Health Morsani College of Medicine, encouraged continued collaboration in the fight to find effective treatments and a cure for Friedreich’s ataxia.
USF’s Dr. Gooch closed the symposium by emphasizing the tremendous progress made in the research and development of lead drug candidates for Friedreich’s ataxia and encouraging all to continue to carry on the fight against the disease with laser focus.
“When pharma becomes engaged, that means the research is good enough to put smart money behind the disease to get a drug to market,†Dr. Gooch said. “There is more than just a glimmer of hope. We’re on the cusp of great possibilities… We look forward to the day when Friedreich’s ataxia will become a historical footnote like smallpox.â€
For more information on the FARA patient registry, which provides notices about new clinical trials, go to http://www.curefa.org/patient-registry

Dr. Gooch, second from left, moderated the question and answer session with patients, l to r, Jade Perry, Erin O’Neil, Kendall Harvey, and Chris Nercesian.

Kyle Bryant with Sam Bridgman, who recently received a full scholarship to attend the graduate program at the USF Muma College of Business.

L to R: FARA President Ron Bartek; Dr. Clifton Gooch, chair of USF Health Neurology; Dr. Jodi Cook, vice president of operations, Agilis Biotherapeutics; Dr. Robert Molinari, founder and CEO of Retrotope; Dr. Jeffrey Sherman, CMO, Horizon Pharma; Dr. Theresa Zesiewicz, director, USF Ataxia Research Center; and Dr. Colin Myer, CMO, Reata Pharmaceutical.
Photos by Eric Younghans, video by Sandra C. Roa, USF Health Communications and Marketing
]]>
]]>
Pharma, biotech leaders will discuss several new clinical studies testing drugs and gene therapy for Friedreich’s ataxia
//www.youtube.com/watch?v=EIMrtGI8Kfo
Tampa, FL (Aug. 31, 2015) — The University of South Florida (USF) will again bring together leading researchers and patients searching for a treatment for Friedreich’s ataxia and related disorders at the seventh annual scientific symposium “Understanding Energy for A Cure.â€Â The symposium will be held 5 to 8:30 p.m., Thursday, Sept. 17, at the USF Marshall Student Center Ballroom, USF Cedar Circle, Tampa, FL 33620.
The event, free and open to the public, is hosted by the Friedreich’s Ataxia Research Alliance (FARA) and the USF Ataxia Research Center.
For the first time, the symposium will include a panel discussion with several biotechnology and pharmaceutical industry leaders about new clinical trials testing drugs and gene therapy for Friedreich’s ataxia. The panelists include representatives from Agilis Biotherapeutics, LLC; Horizon Pharma, plc; Reata Pharmaceuticals Inc; and Retrotope, Inc. The companies collaborate with FARA and academic institutions to focus on research that will improve the quality and length of life for those diagnosed with Friedreich’s ataxia and lead to treatments that eliminate symptoms.
Patients with Friedreich’s ataxia and their families come to USF from across the country to share their thoughts and perspectives about energizing the search for a cure. The event will also be attended by supporters of the FARA Energy Ball gala, held on Saturday, Sept. 19.
The scientific symposium will again be broadcast through Ustream’s CureFA channel, with opportunities for visitors to join the conversation long distance. To watch the presentations in real-time, visit http://www.ustream.tv/channel/curefa on Sept. 17 at 6 p.m. EST. A Ustream account/membership is not needed to join.
Friedreich’s ataxia is a rare, progressive neurodegenerative disease affecting children and adults for which there is currently no approved therapy. Symptoms include neurologic, cardiac, orthopedic, and endocrine dysfunction.
The symposium will be hosted by Clifton Gooch, MD, professor and chair of the Department of Neurology in the USF Health Morsani College of Medicine. Theresa Zesiewicz, MD, professor of neurology and director of the USF Ataxia Research Center, will update attendees on the Friedreich’s ataxia initiatives at USF, one of 10 sites in the international FARA Collaborative Clinical Research Network.
FARA President Ron Bartek and Jennifer Farmer, FARA Executive Director, will give an overview of progress nationwide in Friedreich’s ataxia research.
Featured speaker Sanjay Bidichandani, MBBS, PhD, chair of pediatric medical genetics and professor of pediatrics and biochemistry and molecular biology at the University of Oklahoma College of Medicine and member of the FARA Board of Directors, will talk about the pipeline of investigational treatments for Friedreich’s ataxia.  He was part of the group that discovered the gene for Friedreich’s ataxia and, over the last 15 years, his research has helped characterize the disease’s genetic and epigenetic defect.
USF Health’s Dr. Gooch will moderate a question-and-answer session on patients’ perspectives of living with Friedreich’s ataxia and clinical trial participation.
For more information, please visit http://www.curefa.org/energyball, or call (813) 974-5909.
– About USF Health –
USF Health’s mission is to envision and implement the future of health. It is the partnership of the USF Health Morsani College of Medicine, the College of Nursing, the College of Public Health, the College of Pharmacy, the School of Physical Therapy and Rehabilitation Sciences, and the USF Physicians Group. USF Health is an integral part of the University of South Florida, a high-impact, global research university dedicated to student success. For more information, visit www.health.usf.edu
– About The Friedreich’s Ataxia Research Alliance (FARA) –
FARA is a non-profit organization dedicated to curing FA through research. FARA grants and activities provide support for basic and translational FA research, pharmaceutical/biotech drug development, clinical trials, and scientific conferences. For more information, go to www.curefa.org.
Media contact:
Anne DeLotto Baier, USF Health Communications
abaier@health.usf.edu or (813) 974-3303
Video by Sandra Roa, USF Health Communications
]]>
]]>
The U.S. Food and Drug Administration yesterday designated fast-track status to EPI-743 for the treatment of Friedreich’s ataxia – a move that will help accelerate clinical development of the investigational drug, currently being tested in a multisite, double-blind, placebo-controlled trial led by the University of South Florida.
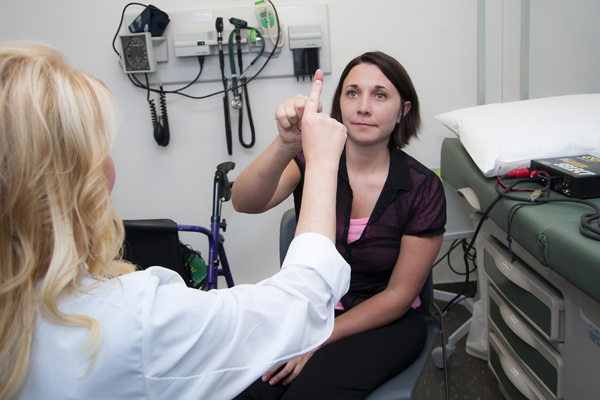
The phase 2b clinical trial of EPI-743 in adults with Friedreich’s ataxia, sponsored by Edison Pharmaceuticals, Inc., in collaboration with the Friedrich’s Ataxia Research Alliance, has been underway since early 2013 at the USF Health, the Children’s Hospital of Philadelphia and the University of California in Los Angeles. Neurologist Theresa Zesiewicz, MD, director of the USF Ataxia Research Center, is the lead investigator for the national trial.
Researchers are primarily testing the effectiveness of EPI-743, a potent antioxidant, on vision, in patients with Friedreich’s ataxia, many of whom experience varying degrees of visual changes. Secondarily, the study is evaluating neurological function.
For more on the fast track status of EPI-743, go to:Â http://www.firstwordpharma.com/node/1195801#axzz2wKgT2a1W
]]>
]]>
First patient enrolled to evaluate investigational drug’s effectiveness  in rare Friedreich’s ataxia genotype
Mountain View, CA; Downingtown, PA; & Tampa, FL (Nov. 4, 2013) — Edison Pharmaceuticals, the Friedreich’s Ataxia Research Alliance (FARA) and the University of South Florida (USF) today announced the initiation of a phase 2 study entitled, “Phase 2A Clinical Trial of EPI-743 on Visual Function in Friedreich’s Ataxia Patients with Point Mutations.â€
Given the rarity of the Friedreich’s ataxia point mutation genotype, the trial is a single-arm, subject-controlled study lasting three months, followed by a three-month extension phase.  Study participants must be between the ages of 18 and 65, possess genetic confirmation of Friedreich’s ataxia point mutation defect, and meet certain disease severity criteria. The primary endpoint of the trial is visual function, with secondary endpoints including neurological and neuromuscular function, and disease-relevant biomarkers.  More information on study specifics is available on ClinicalTrials.gov.
This trial will be conducted at USF Health – a member of the Collaborative Clinical Research Network in Friedreich Ataxia– and is funded by a grant awarded by the Friedreich’s Ataxia Research Alliance. The study sponsor is USF, and the principal investigator is Theresa A. Zesiewicz, MD, FAAN, professor of neurology and director of the USF Ataxia Research Center.
“FARA has been eager to see drug development efforts extend into ultra-rare subgroups of Friedreich’s ataxia, such as those harboring point mutations,†said FARA President Ronald Bartek. “This is the first study dedicated to examining safety and investigational drug response in adults with point mutations in the frataxin gene that represent approximately 2 to 4 percent of the Friedreich’s ataxia patients.â€
EPI-743 is currently being evaluated in a phase 2B placebo-controlled Friedreich’s ataxia trial in individuals with the more common triplet repeat expansion defect in frataxin synthesis.
Friedreich’s Ataxia
Friedreich’s ataxia is an autosomal recessive nuclear DNA inherited mitochondrial disease, affecting an estimated 1 in 30,000 individuals in the United States and Europe. Friedreich’s ataxia is caused by a defect in the gene frataxin, which encodes a 210 amino acid protein that participates in iron-sulfur (Fe-S) cluster protein assembly. As the majority of these Fe-S cluster proteins are localized in the respiratory chain in the mitochondria, patients with Friedreich’s ataxia present with “energy failure†symptoms including ataxia, muscle weakness, heart failure, diabetes, and visual, speech, and hearing deficiencies. Friedreich’s ataxia is a highly debilitating and life-shortening disease and is a member of a larger family of diseases called mitochondrial disease that share as a common biochemical mechanism defects in cellular energy metabolism. There are no FDA-approved drugs for Friedreich’s ataxia.
EPI-743
EPI-743 is an orally bioavailable small molecule being developed by Edison Pharmaceuticals for the treatment of Friedreich’s ataxia and other inherited mitochondrial diseases.
Edison Pharmaceuticals
Edison Pharmaceuticals is a biotechnology company dedicated to developing treatments for children and adults with mitochondrial diseases. More information can be found at www.edisonpharma.com.
FARA
The Friedreich’s Ataxia Research Alliance (FARA) is a national, public, 501©(3), non-profit, tax-exempt organization dedicated to curing Friedreich’s ataxia (FA) through research. FARA grants and activities provide support for basic and translational FA research, pharmaceutical/ biotech drug development, clinical trials, and scientific conferences. FARA also serves as a catalyst, between the public and scientific community, to create worldwide exchanges of information that drive medical advances. For more information about FARA, visit them online at www.curefa.org.
USF Health
USF Health is an integral part of the University of South Florida, a global research university ranked 50th in the nation by the National Science Foundation for both federal and total research expenditures among all U.S. universities. USF Health’s mission is to envision and implement the future of health. It is the partnership of the USF Health Morsani College of Medicine, the College of Nursing, the College of Public Health, the College of Pharmacy, the School of Biomedical Sciences and the School of Physical Therapy and Rehabilitation Sciences; and the USF Physician’s Group. For more information, visit www.health.usf.edu.
]]>
]]>
A record crowd of more than 200 attended the fifth annual Scientific Symposium hosted Sept. 5 by USF and the Friedreich’s Ataxia Research Alliance  (FARA).
There was standing room only as clinicians, researchers, patients and their families and friends gathered at the USF Health Center for Advanced Medical Learning and Simulation to learn about the latest advances in ataxia research. The live audience was bolstered by those from across the world who viewed the symposium livestream through the FARA Facebook page — more than 450 views were recorded.
Many symposium attendees were among the more than 800 friends of the USF Ataxia Research Center and FARA who attended the annual FARA Energy Ball Saturday, Sept. 7, raising more than $1.7 million in donations and in-kind gifts for both organizations.

The collective efforts are producing results. This past year, FARA helped hundreds of patients become part of four new treatment trials, including the Edison Pharmaceutical-sponsored national EPI-743 trial led by USF neurologist Dr. Theresa Zesiewicz, director of the USF Ataxia Research Center. Findings from stem cell and gene therapy studies are giving researchers a better understanding of Friedreich’s ataxia in the drive toward a first treatment, and ultimately a cure, for the progressive, life-limiting neuromuscular disease.
Here are some highlights from this year’s symposium speakers:

Mirella Dottori, PhD, principal investigator and senior research fellow at the Centre for Neuroscience Research, University of Melbourne, Australia. Using skin cells from Friedreich’s ataxia patients converted into pluripotent stem cells, Dr. Dottori’s team recently induced these stem cells to generate the specific cell types that degenerate in Friedreich’s ataxia, including heart and nerve cells.
“In the stem cell field, we have to work on all aspects. It’s critical that we still improve methods of generating patient-derived stem cells. It’s critical that we work on how we move from a stem cell the end-cell lineage that we need. It’s critical to work on how to use these stem cells for drug discovery. It’s critical to understand how to correct a mutation, ultimately for the transplantation of stem cells. A lot of research groups…are all working together on these different aspects to bring everything together. What we’re aiming for, always, is a cure — if not at least a treatment — and I think it’s feasible with all these approaches.â€
Theresa Zesiewicz, MD, professor of neurology and director of the USF Ataxia Research Center, who reported on five clinical trials the center is conducting for patients with FA.Â
“This is a symposium where we come together as community, and it’s important that we all come together as one. Because, as (FARA’s) Ron Bartek said, alone we can do nothing, but together we can do everything.â€

Guy Miller, MD, PhD, CEO of Edison Pharmaceuticals, Inc. Dr. Miller spoke about Edison’s quest for the first successful neuroprotective drug, including lessons learned in the development of the powerful antioxidant EPI-743 for Friedreich’s ataxia, now in a national Phase 2B clinical trial.
“The truth is we have lots of failure in drug development for neurogenerative disease. This process is no easier than a landing on the moon challenge…The first step is critical, and we believe that Friedreich’s ataxia is going to be the disease that sets forth in motion the key learning.â€
“The ability to sequence your genes and understand what errors in DNA might lead to disease, the proteins that come from that, and ultimately the metabolites that regulate those proteins. We’re (Edison) interested in how that whole choir sings together, and we call that redox control.â€
 Â
Â
Jennifer Farmer, FARA executive director
“We hope those of you present here feel the momentum and progress toward changing the future of FA that Ron (Bartek) and I see.â€
Ron Bartek, FARA president
“We need to continue to work together, with your participation and generous support… to push these clinical trials across the finish line – to get that first approved treatment that is so important.â€

Clifton Gooch, MD, professor and chair of the USF Department of Neurology
“What we’ve seen in the five-year trajectory of this symposium is a locomotive gaining steam… We’ve entered the era of a therapeutic revolution in Friedreich’s ataxia… We’re at a great threshold… What we do (today) to improve the lives of patients with FA and make strides toward eradicating this disease will be remembered in the future. We need you, the patients and families, to continue to fight the good fight with us, but some day, I’m convinced, we will win this battle.â€
 Â
Â
Lealan LaRoche, diagnosed with Friedreich’s ataxia at age 20. LaRoche spoke as part of a patient panel discussion moderated by Dr. Gooch. She holds master’s degrees in architecture and city planning.
“Being diagnosed with this debilitating disease made me realize that designing for people who are disabled and abled together could be a great thing.â€

Davis Johnson, diagnosed with FA at age 40. Johnson, a patient on the panel, participated in multiple sports and played football in college. He has a 10-year-old son.
“My diagnosis has made me more patient. I used to live life to the fullest, go places, travel. Now I have to think about getting dressed, eating…everything I do requires some help…. But, being part of the studies and this symposium, and seeing all the people trying to find a cure, gives me hope.â€

Participating in the patient discussion of living with Friedreich’s ataxia were, left to right, Natchez Hanson, Davis Johnson, Lealan LaRoche and Kyle Bryant.
Within the past five years, the multidisciplinary USF Ataxia Research Research Center has become one of the busiest clinical trial centers worldwide for new and experimental approaches to Friedreich’s and other ataxias.
The clinical trial testing the potential Friedreich’s ataxia drug EPI-746 has enrolled its full complement of 60 patients at three sites – UCLA and Children’s Hospital of Philadelphia as well as USF. Results of the trial are expected to be presented at next year’s symposium.

Staff and volunteers from the Friedreich’s Ataxia Research Alliance respond to queries from the online audience watching the symposium via livestream.

Symposium participant Regina Russo shows some muscle in her support of the fight against Friedreich’s ataxia.


Photos by Eric Younghans, USF Health Communications
Â
]]>
]]>
Natchez Hanson considers one of her proudest accomplishments walking across the stage to receive her college diploma – earning a bachelor’s degree in math education.  Hansen, 24, now a high school math teacher in Polk County, lives daily with the challenges of Friedreich’s ataxia.
Friedreich’s ataxia, a rare debilitating neuromuscular disease, typically strikes between the ages of 5 and 15, causing vision, balance, speech and cardiac problems and progressively robbing a young person of their energy, strength and ability to walk.
While physical therapy helps Hanson work on core strength and balance, there is no approved treatment for Friedreich’s. That’s why she is so excited to be part of a USF-led national clinical trial of a drug that researchers, clinicians and patents hope will be the first to improve the symptoms of the life-shortening disease.

USF Health neurologist Dr. Theresa Zesiewicz, left, with patient Natchez Hanson, one of the participants in the USF-led national clinical trial for a potential Friedreich’s ataxia drug.
“I don’t want anyone else to feel the way I did (when I was diagnosed) if they don’t have to,†Hanson said. “I just cried because it was scary. A lot of people died being really young, and I was only 17.  I had all these dreams… I just wanted to be normal.â€
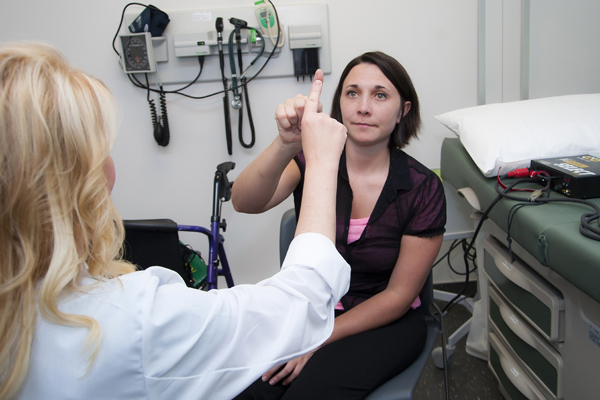
The double-blind, placebo-controlled trial, sponsored by Edison Pharmaceuticals, Inc., in collaboration with the Friedreich’s Ataxia Research Alliance (FARA), is led by neurologist Dr. Theresa Zesiewicz, director of the USF Ataxia Research Center.
Researchers are primarily testing the effectiveness of the investigational drug EPI 743, a potent antioxidant, on vision, in patients with Friedreich’s ataxia, many of whom experience varying degrees of visual changes. Secondarily, the study will evaluate neurological function.
Sixty patients with a genetically-confirmed Friedreich’s ataxia diagnosis have been enrolled in the study, which involves Children’s Hospital of Philadelphia at the University of Pennsylvania and UCLA in Los Angeles, CA, as well as lead site USF.
When the study was announced at last year’s USF/FARA Scientific Symposium, the news was welcomed with cheers and tears by patients and their families who had come to learn about the latest advances in ataxia research.
The reaction and subsequent overwhelming international interest by prospective trial participants did not surprise Dr. Zesiewicz and others who care for those with Friedreich’s ataxia.
“These are young people – children, teens, young adults – affected by a relentless disease that can cause early death,†said Dr. Zesiewicz, USF Health professor of neurology. “There is a real sense of urgency to find a first treatment … We’re racing against time here.â€
EPI-743 isn’t the only drug in the pipeline for Friedreich’s ataxia and related disorders, but it has shown promise in some early, limited trials —  including a study showing neurological benefit in children with Leigh syndrome, a mitochondrial disease, like Friedreich’s, that attacks energy metabolism.  And, so far at least, the drug appears to be well tolerated.
EPI-743 is an extremely powerful antioxidant — much stronger than the vitamin E you can get at a drugstore, Dr. Zesiewicz said. “The thinking is that this very powerful antioxidant will work on the energy-producing part of the cell, the mitochondria, to improve symptoms.â€
While Friedreich’s ataxia is the focus of the USF-led study, Dr. Zesiewicz said, “EPI-743 may have implications for other neurodegenerative disorders like Parkinson’s disease and Alzheimer’s disease.â€
USF is one of 10 partners in the FARA Collaborative Clinical Research Network, an international network of centers sharing and resources to advance treatments and clinical research for people with Friedreich’s ataxia.
The USF Ataxia Research Center conducts several studies in addition to the EPI-743 trial, including a clinical study on cardiac dysfunction in Friedreich’s ataxia and another looking for biomarkers to better monitor disease progression.
Edison Pharmaceuticals CEO Dr. Guy Miller, said USF was the logical choice to take the lead role in the latest EPI-743 trial.
“Many centers have silos of excellence in basic science, or clinical research, or translational research, but at USF there is a cross-section of excellence in all three, which is essential for conducting a well-run 2B clinical trial. †Dr. Miller said. “You also have a fantastic alignment of physician leaders who are innovative and care deeply about patients.â€

L to R: Dr. Stephen Klasko and Dr. Zesiewicz with Dr. Guy Miller, CEO of Edison Pharmaceuticals, at last year’s USF/FARA Scientific Symposium, where Dr. Miller announced the EPI-743 clinical trial for Friedreich’s ataxia. Dr. Miller returned to this year’s symposium, giving an update on drug discovery innovation and progress.
Dr. Clifton Gooch, chair of USF Health Neurology and director of the USF Neuroscience Collaborative, emphasized both the rapid rise of the center and the prospects for EPI-743.
“The USF Ataxia Research Center has become a leading international clinical trials center in just a few short years under Dr. Zesiewicz’s dedicated leadership, and we are excited to now be testing one of the most promising drugs yet tried for the treatment of FA,†he said.
Friedreich’s ataxia is a caused by defects in the gene carrying instructions for a protein called frataxin, which leads to diminished energy production in cells, including those of the nervous system and heart. While rare – only one in 50,000 people are affected by the inherited disease – one in 100 carry the gene, many without even being aware of it.
“Mom and Dad carry the Friedreich’s ataxia gene, but neither one knows that they carry the gene, so it’s often a big surprise to the parents when the child is diagnosed,†Dr. Zesiewicz said.
For many children the first sign that something is physically wrong are falls. That’s what Hanson remembers.
“When I was growing up, I always walked a little goofy,†she said. “I would run and fall down, but then I’d just get right back up and start running again.â€
As time progressed she had trouble walking in a straight line, and leaned on friends and family for support, eventually graduating to crutches.  After physicians ruled out several other potential causes, including a brain tumor, Hanson was formally diagnosed with Friedreich’s ataxia at age 17.  She started using a walker two years ago.
At first, Hanson said, she was embarrassed by the walker, but now she embraces the greater mobility it provides and uses its storage compartment to help carry school supplies. “At least no one thinks I’m drunk anymore, so that’s good,†she quips.

Results of the Edison EPI-743 trial are expected later next year. In the meantime, Hanson continues to move forward, refusing to let a disease stop her. For the third consecutive year, she was among the patients who spoke Sept. 5 at the 5th Annual USF-FARA Scientific Symposium — sharing their stories of living as optimistically as possible with Friedreich’s ataxia.
“You only have one life, and everyone has a burden to bear. You either laugh about it or you cry about it – and I’m done crying,†she said. “I’m trying to be as happy as I can be.â€
See related story: USF/FARA scientific symposium to share latest advances in ataxia research

Photos by Aimee Blodgett, USF Communications and Marketing
]]>
]]>
Friedreich’s Ataxia Research Alliance and University of South Florida host Sept. 5th event
//www.youtube.com/watch?v=NWZxaPjI_wo
Tampa, FL (August 28, 2013) – Leading scientists and clinicians searching for a treatment for Friedreich’s ataxia (FA) and related disorders will gather for the fifth annual scientific symposium “Understanding a Cure,†6 to 8 p.m., on Thursday, Sept. 5, at the USF Health Center for Advanced Medical Learning and Simulation, or CAMLS, 124 South Franklin Street, Tampa, FL 33602.
The symposium, free and open to the public, is hosted by the Friedreich’s Ataxia Research Alliance (FARA) and the University of South Florida (USF) Ataxia Research Center. Speakers will share the latest advances in research — from harnessing stem cell technology to investigating the effectiveness of a new medication.
The event routinely draws patients with Friedreich’s ataxia and their families from across the country to share their thoughts and perspectives about energizing the search for a cure. The event will also be attended by supporters of the FARA Energy Ball, held on Saturday, Sept. 7.
For the second year in a row, the scientific symposium will be broadcast through the FARA Facebook page, with opportunities for visitors to join the conversation long distance. To watch the presentations in real-time, visit https://www.facebook.com/CureFA/app_196506863720166 on Sept. 5 at 6 p.m. EST. A Facebook account/membership is not needed to join.
Friedreich’s ataxia is a rare, debilitating neuromuscular disorder. Symptoms, emerges either between ages 5 and 15 or in adulthood and can progress to severe disability and include the following: loss of coordination and muscle weakness that leads to wheelchair use, energy deprivation and fatigue, vision impairment, hearing loss, slurred speech, aggressive scoliosis, diabetes, and life-shortening cardiac disease. There is not yet an approved treatment or a cure.
At last year’s symposium, Guy Miller, MD, PhD, CEO of Edison Pharmaceuticals Inc., announced that USF would lead a multisite study testing the safety and effectiveness of a potent antioxidant, the investigational drug known as EPI-743, for patients with Friedreich’s ataxia. Dr. Miller will return this year to report on the progress of that ongoing Edison-funded clinical trial, led by Theresa Zesiewicz, professor of neurology at USF Health.
Dr. Zesiewicz will join the discussion about the EPI-743 trial and update attendees on several other studies being conducted by the USF Ataxia Research Center.
Symposium speakers will include Mirella Dottori, PhD, principal investigator and senior research fellow at the Centre for Neuroscience Research, University of Melbourne, Australia, who will talk about //www.youtube.com/watch?v=TnLGtN8RVR4
Friedreich’s ataxia treatments with stem cells. Using skin cells from Friedreich’s ataxia patients converted into pluripotent stem cells, Dr. Dottori’s team recently induced these stem cells to generate the specific cell types that degenerate in Friedreich’s ataxia, including heart and nerve cells.
FARA President Ron Bartek and Jennifer Farmer, FARA executive director, will address progress nationwide in the research and management of Friedreich’s ataxia.
Dr. Zesiewicz will moderate a question-and-answer session on patients’ perspectives of living with ataxias, and Clifton Gooch, MD, chair of neurology at USF Health, will provide closing remarks.
USF is one of 10 sites included in FARA’s Collaborative Clinical Research Network, an international network of centers that share data and resources to advance treatments and clinical research for people with Friedreich’s ataxia.
For more information, please visit http://www.curefa.org/energyball/sep5.html or call (813) 974-5909.
– About USF Health –
USF Health’s mission is to envision and implement the future of health. It is the partnership of the USF Health Morsani College of Medicine, the College of Nursing, the College of Public Health, the College of Pharmacy, the School of Biomedical Sciences and the School of Physical Therapy and Rehabilitation Sciences; and the USF Physician’s Group. The University of South Florida is a global research university ranked 50th in the nation by the National Science Foundation for both federal and total research expenditures among all U.S. universities. For more information, visit www.health.usf.edu
    – About The Friedreich’s Ataxia Research Alliance (FARA) –
FARA is a non-profit organization dedicated to curing FA through research. FARA grants and activities provide support for basic and translational FA research, pharmaceutical/biotech drug development, clinical trials, and scientific conferences. For more information, go to www.curefa.org.
Video produced by USF Health Communications
Â
]]>







