]]>
The new study, with collaboration from USF Health, establishes potential targets for treating diabetic damage to nerves regulating heart rate
Cardiac autonomic neuropathy, a common complication of diabetes, can increase the risk of severe heart disease, and sudden cardiac death caused by abnormal heart rhythms known as arrhythmias. Â Earlier research at the USF Health Morsani College of Medicine, University of South Florida, and elsewhere has helped define how diabetes affects innervation of the heart and damages nerves that control involuntary (autonomic) regulation of body functions, including heart rate.
Now a new preclinical study, including USF Health collaborator Sami Noujaim, PhD, has discovered that inhibiting the enzyme glycogen synthase kinase-3B (GSK3ß) in heart muscle cells reduces dysfunctional parasympathetic nervous system activity associated with diabetes. Recently published in PLOS ONE, the Tufts University-led study used a mouse model for type 1 diabetes (Akita mouse) in which the cardiac-specific GSK3ß gene was selectively and partially inactivated.
In particular, the findings support a new mechanism for understanding how overstimulation of GSK3ß in type 1 diabetes affects development of an abnormal cardiac parasympathetic response in diabetes and also suggests new targets for treating or preventing damage to nerves that regulate heart rate.
The sympathetic nervous system, which mobilizes the body’s “fight-or-flight†responses to stress, accelerates heart rate. The “rest-and-digest†parasympathetic nervous system, which helps calm the body, slows heart rate.
These two components of the involuntary nervous system are normally in constant flux. In fact, slight variations in the time between each heartbeat, known as heart rate variability, characterizes a healthy heart in which both sympathetic and parasympathetic systems communicate effectively to maintain regulatory balance. Conversely, in cardiovascular disease and diabetes, heart rate variability decreases.
“Reduced heart rate variability is a harbinger of bad cardiovascular outcomes in the future,†said co-investigator Dr. Noujaim, associate professor of molecular pharmacology and physiology at USF Health. “So, we want to find a signaling pathway to target that can increase heart rate variability. Our goal is to reduce the likelihood of the diabetic heart developing poor cardiovascular outcomes, including arrhythmias.â€
Dr. Noujaim directs a cardiac electrophysiology research laboratory, which has the tools to translate mouse heart activity into frequencies used to analyze beat-to-beat variations in heart rate. High frequencies are predominately generated from parasympathetic regulation of heart rate, while low frequencies result largely from sympathetic regulation.
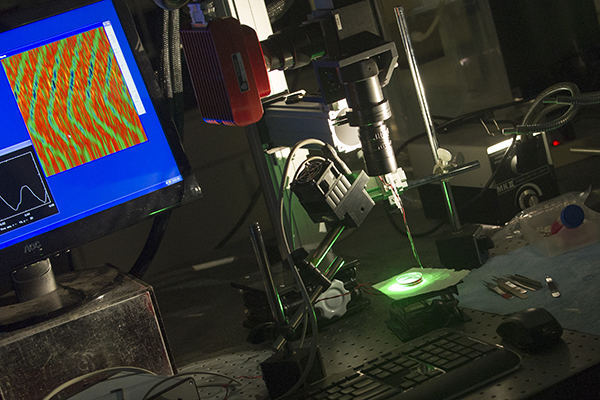
Dr. Noujaim’s laboratory uses specialized equipment to measure the electrical activity of heart muscle cells, including those affected by diabetes.
Among some key findings of the PLOS ONE study:
- Decreasing the expression of GSK3ß in the hearts of the Akita mice reversed cardiac parasympathetic dysfunction. It also increased (improved) parasympathetic response, as measured by the increased high-frequency proportion of heart rate variability.
- Reducing GSK3ß expression also increased activity of IKACH in heart muscle cells of the diabetic mice. The parasympathetic nervous system slows down heart rate through activation of the IKACH potassium current in the cardiac cells. However, Dr. Noujaim said, when GSK3ß is upregulated in response to high glucose levels in the diabetic heart, it causes the suppression of the IKACH current. When IKACH is suppressed, the parasympathetic tone does not reach the heart, thereby allowing the heart-rate accelerating sympathetic nervous system tone to dominate and heart rate variability to decline, he added.
- Collectively, the series of experiments demonstrate that increased GSK3ß activity amplifies cardiac parasympathetic malfunction in type 1 diabetes through regulation of IKACH.
“The data further establish GSK3ß and the GSK3ß signaling pathway as potential therapeutic targets in the treatment and prevention of autonomic dysfunction in diabetic patients,†the study authors conclude.
The research was supported by the Juvenile Diabetes Research Foundation and National Institutes of Health grants.
]]>
]]>
The University of South Florida-led study created a rigorous mathematical model to simulate maternal and newborn outcomes, comparing elective induction to expectant management
TAMPA, Fla. (April 25, 2018) – It’s better to induce labor than to watch and wait. That’s the main result of a new study published in PLOS ONE.
Researchers at the University of South Florida Morsani College of Medicine found that first-time healthy mothers electively induced at 39 weeks of pregnancy are at lower risk of cesarean delivery and other serious complications compared to those expectantly managed and induced at 41 weeks if undelivered by then.
Obstetricians generally recommend inducing (artificially stimulating) labor and delivery after 41 weeks since continued pregnancy after this point is associated with higher chances of stillbirth and risks to the mother. However, uncertainty exists about best timing of deliveries when the fetus is between 39 and 41 weeks. The USF-led study presents compelling evidence that electively inducing labor at 39 weeks results in less risk, not more, to mothers and their newborn infants than expectant management, or watchful waiting, in the same population.
Elective induction was associated with reduced rates of cesarean deliveries and maternal complications including preeclampsia, fewer stillbirths and newborn deaths, and lower rates of newborn complications such as birth injuries, respiratory distress and shoulder dystocia (infant’s shoulder becomes lodged behind the mother’s pubic bone).
“Safely preventing primary cesarean deliveries, stillbirths and reducing other perinatal complications are of the greatest concern,†said the study’s principal investigator Charles J. Lockwood, MD, senior vice president for USF Health and dean of the Morsani College of Medicine. “Sometimes clinicians do something because that is the way it’s always been done. These findings demonstrate the importance of strong evidence-based research in informing and shaping standards of care.â€
“When I was in residency training, it was drilled into us that elective induction of labor increased C-section deliveries. But, actually we found the opposite†said lead author Rachel Sinkey, MD. Dr. Sinkey conducted the research when she was a maternal-fetal medicine fellow at USF Health and is now an assistant professor of obstetrics and gynecology in the Division of Maternal-Fetal Medicine, University of Alabama at Birmingham.
The USF researchers created a rigorous mathematical model, using data from the NIH’s Safe Labor Consortium supplemented by review of the latest medical literature. A theoretical cohort of 100,000 patients was used to simulate key pregnancy outcomes for two groups: first-time mothers with low-risk pregnancies who elected to have labor induced at 39 weeks and those choosing expectant management with induction of labor only if medically indicated or if their pregnancies extended beyond 41 weeks.
Compared to elective induction of labor at 39 weeks, waiting to induce labor at 41 weeks resulted in increased:
- C-section rates (35.9 vs. 13.9 percent). Even when the cervix was unfavorable in position or dilation, C-sections were more frequent with expectant management.
- Maternal complications (21.2 vs. 16.5 percent)
- Stillbirths (0.13 vs. 0 percent)
- Newborn deaths (0.25 vs. 0.12 percent)
- Severe neonatal complications (12.1 vs. 9.4 percent)
The USF findings were recently corroborated by results of the National Institutes of Health-supported ARRIVE trial of induction versus expectant management. That large-scale, randomized controlled trial also found that induction of labor at 39 weeks in low-risk pregnant women resulted in a lower frequency of cesarean deliveries and preeclampsia.
The USF findings are supported by biologically plausible explanations. Both inadequate delivery of essential nutrients and oxygen to the fetus, known as placental insufficiency, and increasing fetal growth are associated with advanced pregnancies and may explain why elective induction of labor at 39 weeks reduces the risk of adverse pregnancy outcomes, Dr. Sinkey said.
More study is needed to address health care system logistics and costs associated with routine elective induction of labor at 39 weeks. Spontaneous labor without medical intervention remains critically important to many women, Dr. Sinkey said, however, induction of labor should be presented to patients as an acceptable option.
“We acknowledge that not all women nor their providers desire elective inductions and we recommend that the patient should be final arbiter of the timing and mode of delivery after adequate counseling and informed consent,†the study authors conclude.
]]>
]]>
The USF-led study may lead to drug therapies to replenish cells destroyed or damaged by diabetes
Tampa, FL (Sept. 23, 2015) — Cells that express neurogenin 3 (NGN3) may one day be harnessed to create a plentiful supply of insulin-producing beta cells for the treatment of diabetes, a study led by researchers at the University of South Florida suggests.
NGN3 is the master gene driving development of the human endocrine pancreas, including the beta cells that make and secrete the hormone insulin, which helps control blood sugar levels. In type 1, or juvenile, diabetes, insulin-producing beta cells are destroyed by the person’s immune system, and patients need insulin injections to survive. Patients with the more common type 2 diabetes, referred to as adult-onset diabetes, produce insulin but their bodies cannot use it properly, and they often require extra insulin.
In a study recently published in the journal PLOS ONE, researchers from the Children’s Research Institute, USF Health Morsani College of Medicine; Johns Hopkins University School of Medicine; and the University of Illinois at Chicago, detected the NGN3 protein in histologically normal pancreatic biopsies from two sources — cadavers and patients requiring biopsy for diagnostic purposes.
“NGN3 expression in the adult pancreas was unexpected, because it cannot be detected in the adult rodent pancreas – only during fetal development,†said the study’s principal investigator Michael Shamblott, PhD, an endowed chair of pediatrics at the Children’s Research Institute, USF Health Morsani College of Medicine, whose research focuses on regenerative cell therapies to replenish the insulin-producing cells destroyed or damaged by diabetes.
The researchers found NGN3-expressing cells in the exocrine pancreas, the part of the pancreas that produces digestive enzymes. NGN3-expressing cells closely match the characteristics of both mouse and human endocrine “progenitor†cells that give rise to all and only cells in pancreatic tissue known as islets. These cells can be collected from cadaveric pancreas tissue or from the patient’s own pancreas and coaxed to resemble beta cells that produce and secrete insulin.
Islet transplantation as a means of reversing diabetes has been hampered by the limited durability of grafts and scarcity of pancreas donors. It can take multiple donors to yield enough islet cells for one recipient and donor tissue is not matched to the recipient so strong immunosuppressive drugs are required to avoid rejection.
“Now that we know these NGN3 cells are a normal part of adult human pancreas biology, we can learn to increase them and to coax them towards becoming differentiated pancreatic endocrine cells by using specific drugs,†Dr. Shamblott said. “Our goal is to regenerate functional beta cells that can cure diabetes.â€
The researchers have also discovered several pathways regulating NGN3 in the adult pancreas, which may be targets of future drug treatments of diabetes, Dr. Shamblott said.
Article citation:
Danielle L. Gomez, Marci O’Driscoll, Timothy P. Sheets, Ralph H. Hruban, Jose Oberholzer, James J. McGarrigle, Michael J. Shamblott, “Neurogenin 3 Expressing Cells in the Human Exocrine Pancreas Have the Capacity for Endocrine Cell Fate,†PLOS ONE, Aug. 19, 2015, DOI: 10.1371/journal.pone.0133862.
About USF Health
USF Health’s mission is to envision and implement the future of health. It is the partnership of the USF Health Morsani College of Medicine, the College of Nursing, the College of Public Health, the College of Pharmacy, the School of Physical Therapy and Rehabilitation Sciences, and the USF Physician’s Group. The University of South Florida is a Top 50 research university in total research expenditures among both public and private institutions nationwide, according to the National Science Foundation. For more information, visit www.health.usf.edu
Media contact:
Anne DeLotto Baier, USF Health Communications and Marketing
abaier@health.usf.edu or (813) 974-3303
]]>
]]>
The mouse model study combined a ketogenic diet and supplements with hyperbaric oxygen therapyÂ
Tampa, FL (June 10, 2015) — A team of researchers from the Hyperbaric Biomedical Research Laboratory at the University of South Florida (USF) has doubled survival time in an aggressive metastatic cancer model using a novel combination of non-toxic dietary and hyperbaric oxygen therapies.
The study, “Non-toxic metabolic management of metastatic cancer in VM mice: Novel combination of ketogenic diet, ketone supplementation, and hyperbaric oxygen therapy,†was published online today in PLOS ONE.
Led by principal investigator Dominic D’Agostino, PhD, assistant professor in the Department of Molecular Pharmacology and Physiology at the USF Health Morsani College of Medicine, the recently published research shows the beneficial effects of using ketone supplements in conjunction with a non-toxic therapeutic regimen developed previously by the team. Ketones are produced when the body begins burning fat instead of carbohydrates for energy.

Principal investigator Dominic D’Agostino, PhD, and research associate Angela Poff, PhD, measure tumor growth in the mice receiving the investigational treatment in USF’s Hyperbaric Biomedical Research Laboratory
The research group previously published a study in PLOS ONE demonstrating the anti-cancer effects of therapeutic ketosis induced by the high-fat, low-carbohydrate ketogenic diet (KD) combined with hyperbaric oxygen therapy (HBOT), which involves breathing high-pressure oxygen. Inducing therapeutic ketosis solely with the ketogenic diet can be difficult, however, so the USF researchers created novel metabolic agents that induce ketosis without dietary restriction. These ketone supplements slowed cancer growth on their own, and further enhanced the combined therapeutic effects of KD and HBOT.
In the recent USF study, mice with advanced metastatic cancer were fed either a standard high-carbohydrate diet or a carbohydrate-restricted ketogenic diet with ketone supplements and HBOT. Therapeutic ketosis causes the body to shift from using glucose to fatty acids and ketones bodies for energy.
Normal healthy cells readily adapt to using ketone bodies for fuel, but most cancer cells lack this metabolic flexibility. Solid tumors also have areas of low oxygen, which promote tumor growth and metastatic spread. HBOT involves breathing 100 percent oxygen at elevated barometric pressure, saturating the tumors with oxygen. When administered properly, both ketosis and HBOT are non-toxic and may even protect healthy tissues while simultaneously damaging cancer cells.
Animals receiving the combination of KD, ketone supplements, and HBOT lived 103 percent longer than mice fed a standard high-carbohydrate diet. The researchers believe their study demonstrates the potential of these non-toxic therapies to contribute to current cancer treatment regimens and significantly improve the outcome of patients with advanced metastatic cancer.
Researchers at USF and elsewhere are investigating the potential benefits of the physiological state of therapeutic ketosis for several major diseases. The USF team believes these novel ketone supplements may be effective in other disorders besides cancer and have ongoing studies to test their potential use in wound healing, epilepsy, amyotrophic lateral sclerosis (ALS), Alzheimer’s disease, glucose transporter type 1 (GLUT1) deficiency syndrome, and exercise performance.
The cancer study, funded by a charitable donation from Scivation Inc., was inspired by the research of Professor Thomas Seyfried of Boston College. Dr. Seyfried has advanced the theory that cancer is a metabolic disease, leading to the development of new strategies to treat and prevent cancer. The USF researchers are currently collaborating with other scientists to explore options for establishing human clinical trials.
-USF Health-
USF Health’s mission is to envision and implement the future of health. It is the partnership of the USF Health Morsani College of Medicine, the College of Nursing, the College of Public Health, the College of Pharmacy, the School of Biomedical Sciences and the School of Physical Therapy and Rehabilitation Sciences; and the USF Physician’s Group. The University of South Florida is a Top 50 research university in total research expenditures among both public and private institutions nationwide, according to the National Science Foundation. For more information, visit www.health.usf.edu
Media contact:
Anne DeLotto Baier, USF Health Communications & Marketing
abaier@health.usf.edu or (813)974-3303
]]>
]]>
Umbilical cord cell and growth factor treatment tested in animal models could offer hope for millions, including U.S. war veterans with traumatic brain injuries

USF Health neuroscientist Cesar Borlongan, PhD, the study’s lead author.
Tampa, FL (March 20, 2014) — Traumatic brain injuries (TBI), sustained by close to 2 million Americans annually, including military personnel, are debilitating and devastating for patients and their families. Regardless of severity, those with TBI can suffer a range of motor, behavioral, intellectual and cognitive disabilities over the short or long term. Sadly, clinical treatments for TBI are few and largely ineffective.
In an effort to find an effective therapy, neuroscientists at the Center of Excellence for Aging and Brain Repair, Department of Neurosurgery in the USF Health Morsani College of Medicine, University of South Florida, have conducted several preclinical studies aimed at finding combination therapies to improve TBI outcomes.
In their study of several different therapies—alone and in combination—applied to laboratory rats modeled with TBI, the USF researchers found that a combination of human umbilical cord blood cells (hUBCs) and granulocyte colony stimulating factor (G-CSF), a growth factor, was more therapeutic than either administered alone, or each with saline, or saline alone.
The study appeared in a recent issue of PLoS ONE.
“Our results showed that the combined therapy of hUBCs and G-CSF significantly reduced the TBI-induced loss of neuronal cells in the hippocampus,†said study lead author Cesar V. Borlongan, PhD, professor of neurosurgery and director of USF’s Center of Excellence for Aging and Brain Repair. “Therapy with hUBCs and G-CSF alone or in combination produced beneficial results in animals with experimental TBI. G-CSF alone produced only short-lived benefits, while hUBCs alone afforded more robust and stable improvements. However, their combination offered the best motor improvement in the laboratory animals.â€
For full story, go to:Â http://www.research.usf.edu/absolute-news/templates/template1.aspx?articleid=2106&zoneid=1
]]>