From designing 3D printed test swabs, to researching antibody responses and engaging in leading clinical trials, USF Health scientists rapidly team up to help fight COVID-19
While the world waits for therapies to reduce death rates and a widely available vaccine to prevent COVID 19, team science at USF Health and other academic medical centers continues to take on an unprecedented sense of urgency.
Globally, scientists across disciplines are publicly sharing their ideas, expertise and data like never before – all singularly focused on finding solutions to a highly contagious and potentially life-threatening new virus known as severe acute respiratory syndrome coronavirus 2, or SARS-CoV-2.
Since the pandemic began, the number of studies posted by researchers worldwide to open-access repositories like bioRxiv and medRxiv has skyrocketed. These preprints – papers written after a study concludes but made available before peer review – let scientists disseminate their findings more quickly and obtain instant feedback on their work. Researchers also continue to identify and share viral genome sequences, protein structures, and COVID-19 related epidemiological and clinical data through online databases.
Meanwhile, thousands of clinical trials have been launched as academic medical centers, hospitals and laboratories join forces with government and industry in the search for optimal diagnostics and therapies. At USF Health, more than 65 COVID-19 related laboratory, clinical and epidemiological projects are underway or in final stages of the approval process. These represent unique research efforts by the faculty of all four USF Health colleges, as well as joint efforts with pharmaceutical firms and biotechnical and software companies. Many of the patient-related studies are conducted by USF Health faculty physicians at Tampa General Hospital. USF Health is also working with Tampa General to create a biorepository that collects, processes and stores health data and residual specimens from patients who test positive or negative for COVID-19 to use in future biomedical research.
“The need for rapid and accurate basic and clinical results has never been greater. The scientific community has risen to the challenge of a lifetime and continues to push forward,” said Stephen Liggett, MD, associate vice president for USF Health Research and vice dean for research in the Morsani College of Medicine. “Without a doubt we are still in the early stages of understanding this new coronavirus – in knowing who should be tested and how often, and which tests work best; in knowing how to treat patients and how effective vaccines will be in conferring immunity.”
View the interview with Stephen Liggett, MD, associate vice president for research at USF Health, who discusses how the COVID-19 pandemic has changed research here.
USF faculty and student researchers have been quick to mobilize their talent and resources, Dr. Liggett said. “They want to do whatever they can to find answers — both to help fight this pandemic and to prepare for future outbreaks.”
How are some key scientific areas contributing to the pandemic response? Below are just a few examples provided by USF Health scientists:
Epidemiology: Containing the spread of the virus
From the start, epidemiologists have been at the forefront of efforts to understand how fast and why SARS-CoV-2 is spreading. Also known as disease detectives or virus hunters, epidemiologists and the models using data they gather are instrumental in tracking and predicting the patterns of disease transmission in populations, said Thomas Unnasch, PhD, distinguished professor in the USF College of Public Health and codirector of the Center for Global Health Infectious Disease Research. Their work had been critical for both guiding policymakers’ plans to curb the pandemic and helping evaluate whether countermeasures to contain the virus are working.
“We’ve been hunkered down in the midst of a pandemic wildfire and testing only the symptomatic people most likely to be infected” — largely to prevent surges of sick patients from overwhelming the health care system, Dr. Unnasch said. “We’re still missing about 90 percent of the population with COVID-19 infections exhibiting mild or no symptoms.”

USF College of Public Health’s Thomas Unnasch, PhD, oversees the COVID-19 symptom surveillance network for Tampa Bay.
Dr. Unnasch oversees a symptom-based surveillance network launched in mid-April to help identify and map COVID-19 hotspots across the Tampa Bay region. USF College of Public Health researchers worked with the Hillsborough, Pinellas, Pasco and Polk County Health Departments to create the Tampa Bay symptom surveillance survey, adapting existing COVID-19 surveillance technology developed by the Puerto Rico Sciences Trust and deployed in Puerto Rico and, more recently, the Boston area through Harvard University.
The anonymous survey asks Tampa Bay residents questions about potential exposure and symptoms consistent with COVID-19. The information collected, which drills down to the zip code level, is provided to the local health departments and hospital groups.
Surveillance – a tool commonly used by public health agencies to identify and prevent the spread of HIV, tuberculosis, anthrax and other infectious diseases – can help fill in the gaps created by limitations inherent in a complex society, such as a lack of uniform testing, Dr. Unnasch said.

COVID-19 cases in Pinellas and Hillsborough County broken down by zip code, as tracked and entered by the Hillsborough County Health Department on April 16, 2020. Pasco and Polk counties have since been added to the symptom surveillance system.
“So far the only way to prevent the disease is to prevent transmission of the virus. That has meant everyone doing the right thing — staying at home, social distancing face masks, and hygiene,” Dr. Unnasch said. “As we reopen our communities, surveillance can help us do that safely by detecting clusters of new cases early at a very targeted level, so we can stomp out the embers before they reignite COVID-19 outbreaks.”
Real-time mapping of suspected COVID-19 hotspots can be used to strategically direct Tampa Bay’s public health resources to specific areas where testing, contact tracing and isolation are most likely needed, he said.
“The more data we get and the more accurate the information, the more powerful the tool will be.”
Biostatisticians: Keeping the bias at bay
The data collected by epidemiologists or other health researchers can be fed into mathematical models that predict how fast COVID-19 infections may spread or the number of deaths expected in an overall population. At the community/clinical level, predictive models can help hospitals and medical staff triage patients and allocate limited health care resources (like ICU beds or ventilators) by estimating the risk of people being infected or having a poor disease outcome.
While they can be useful to prepare for worst-case scenarios, predictive models have differed widely in their forecasts – and sometimes they can cause more harm than benefit in guiding policy or clinical decisions, said Ambuj Kumar, MD, MPH, director of the Research Methodology and Biostatistics Core, USF Health Office of Research.
Dr. Kumar, a biostatistician and associate professor of internal medicine, points to a recently published systematic review analyzing studies of prediction models for the diagnosis and prognosis of patients with COVID-19. This review concluded that all 31 clinical models were poor quality, at high risk of bias, and their reported performance was likely overly optimistic.
Methodologist/biostatisticians like Dr. Kumar are trained to recognize the issues and complications arising from the analysis of human health data. They play a key role in any team designing and executing a model, providing the statistical methodology needed to draw meaningful conclusions or make predictions. These data scientists help reduce bias in selecting sample populations, observing or reporting findings, and measurement. They are attuned to factors that can interfere with an accurate estimate of cause-and-effect.
Requiring frequent updates, projections are only as good as the model’s underlying assumptions and the reliability and standardization of the data applied to the model, Dr. Kumar said.
For instance, the commonly cited Institute for Health Metrics and Evaluation model assumes social distancing and other strong voluntary measures to control viral spread will stay in place, but predicting how people will behave as the U.S. reopens in phases is tricky. And, the death data relied upon by many models may be confounded a lack of consistency in the way COVID-19 deaths are reported and counted by hospitals and health departments. (Public health experts have suggested that deaths are undercounted.)

Predictive modeling uses existing data and reasonable assumptions to forecast how an infectious disease spreads in the real world. As more data becomes available, it triggers adjustment of the model, resulting in different outcomes.
Many people understandably want to know now what to expect during this pandemic: How many more cases? How long will it last? When can I safely return to work, or school? Will there be a second wave?
But, many uncertainties about testing, immunity, susceptibility and treatments still influence the variables that make up the algorithms forecasting COVID-19 outcomes, Dr. Kumar said. As the reliability and accuracy of rapidly accumulating data improves, so should the models, he added.
“Predicting the future is particularly challenging when we’re dealing with a virus new to the entire world,” Dr. Kumar said. “Whether you’re battling COVID-19 or another crisis, you can’t compromise on the systematic, standardized approach needed to create a useful model, or study. If you want accurate results, there’s no substitute for good, rigorous science.”
Virology: Studying how SARS-CoV-2 works
To develop effective therapies and vaccines to combat COVID-19, scientists need to understand how the virus functions, including its interaction with human immune response. That’s the role of virologists like Michael Teng, PhD, associate professor of internal medicine in the USF Health Morsani College of Medicine.
Dr. Teng has spent many years working with the National Institutes of Health and other groups on research and development of a vaccine for respiratory syncytial virus, or RSV. While RSV was discovered over 60 years ago, researchers continue to work on a vaccine for this common respiratory virus that infects virtually every child by age 2.

Like many other scientists, USF Health virologist Michael Teng, PhD, quickly pivoted from his usual research activities to respond to the new global health threat.
Scientists and companies now testing a myriad of SARS-CoV-2 vaccines in the pipeline have benefited from the extensive RSV research, Dr. Teng said. “They’ve learned a lot from RSV about what works and what pitfalls to avoid in vaccine development.”
Like many other scientists, Dr. Teng quickly pivoted from his usual research activities to respond to the new global health threat. In mid-March his laboratory studied the durability and effectiveness of the 3D-printed nasal swabs successfully created for COVID-19 testing by a team at USF Health Radiology and its innovative 3D Clinical Applications Division, directed by associate professor Summer Decker, PhD. Faculty with expertise in anatomy and infectious diseases as well as radiology contributed to the effort. The ambitious 3D design, modeling and printing project teamed USF Health with Formlabs, a 3D printer manufacturer, and Northwell Health, the largest hospital system in New York, the pandemic’s U.S. epicenter.
An integral part of coronavirus test kits that detect the RNA virus’s genetic code, swabs were in extremely short supply as the pandemic escalated. The slender, flexible device collects a sample from the nasal passages or throat, and that sample goes into a test tube containing transport media for preservation until the specimen is processed by a hospital or commercial laboratory. Using RSV as a proxy for a SARS-CoV-2, synthetic respiratory tract mucous (made by USF Health’s Sophie Darch, PhD), and a World Health Organization recipe for transport media, Dr. Teng demonstrated that the 3D-printed alternative swabs worked as well as conventional commercial swabs to safely collect enough of the sample, without leeching into transport media or interfering with the nucleic acid test’s ability to detect virus particles.
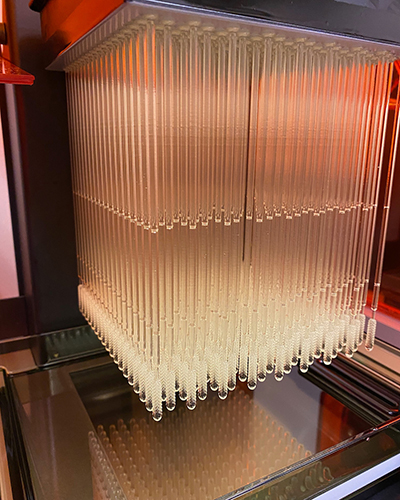
Top: A USF Health Radiology-led team successfully designed, tested and produced a prototype 3D printed nasopharyngeal swab in record time. As of late May, more than 50,000 of the nasal swabs had been mass produced and were being used worldwide by health care providers to alleviate bottlenecks in COVID-19 testing. Bottom: Jonathan Ford, PhD, a biomedical engineer in USF Health Radiology, holds a cube of the 3D diagnostic nasal swabs.
The 3D printed swabs, fabricated with FDA-approved, nontoxic materials, also passed performance benchmarks when clinically validated in hospitalized patients undergoing COVID-19 screening at Tampa General Hospital and Northwell Health sites. (A larger-scale multisite clinical trial, led by USF Health Infectious Disease Division Director Kami Kim, MD, is further evaluating the performance of the investigational 3D swabs for diagnostic testing.) Meanwhile, several hundred hospitals and academic medical centers across the country, many state governments, and international agencies and health care facilities are already using the USF-patented swabs to alleviate bottlenecks in COVID-19 testing.
The team worked late nights, taking only about a week from swab prototype design and bench testing to the start of clinical validation. “That’s an incredibly fast turnaround time,” Dr. Teng said.
Dr. Teng is also a coinvestigator for a Morsani College of Medicine-College of Public Health project led by Dr. Kim, which is working to find and map epitopes, the parts of SARS-CoV-2 proteins recognized by the immune system. Antibodies are made by the immune system in response to a threat from a specific virus, bacteria and other harmful pathogen. Some epitopes are associated with protective antibody responses that neutralize (inactivate) a virus when that pathogen is recognized by the immune system again. Others may actually lead to a harmful immune response when a person is exposed to the same virus a second time. The USF Health team wants to identify specific epitopes triggering strong protective antibodies to help researchers design vaccines that mimic a beneficial immune response against COVID-19.
“The data we gather may also be useful in screening (convalescent) plasma for specific antibodies that may best be used to treat critically ill COVID-19 patients,” Dr. Teng said.

Thomas McDonald, MD, USF Health professor of cardiovascular sciences, is investigating whether genetic, physiological or medication-interaction factors may contribute to racial and ethnic disparities in COVID-19 infection rates and cardiovascular complications.
A coronavirus “pseudotype” created by Dr. Teng’s laboratory is being used by the team working on finding neutralizing antibodies and a second USF Health team investigating what factors may affect who suffers worse COVID outcomes.
The second team, led by Thomas McDonald, MD, professor of cardiovascular sciences at the USF Health Heart Institute, wants to know if socioeconomic differences alone account for racial and ethnic disparities in who gets sicker and dies from COVID-19, or if genetic, physiological, or even medication-interaction factors contribute to disproportionate infection rates and cardiovascular complications.
Human pluripotent stem cells grown in Dr. McDonald’s laboratory are prodded to become lung, immune, and heart cells in a petri dish. The stem cells come from blood samples donated by many patient volunteers of different ages, genders and races, as well as various pre-existing cardiovascular conditions. These tissue samples will be infected with the COVID-19 proxy virus engineered by Dr. Teng.
The substitute virus combines the well-studied vesicular stomatitis virus (VSV) with an outer shell containing the spike protein on the surface of SARS-CoV-2 that allows the coronavirus to enter human cells. This non-replicating virus is “a sheep in wolf’s clothing,” invading cells like the COVID-19 virus without harming scientists working with the pathogen, Dr. McDonald said. VSV also expresses the same enzyme, luciferase, that gives fireflies their glow. When hit with a chemical, this “firefly luciferase” lights up the virus so researchers can trace how much invades cells and which cell types are vulnerable.
“With a machine we can image the range of light, which is the level of infection coming out of the cells,” Dr. Teng said.
For the Dr. Kim-led study evaluating the ability of different serum antibodies to block the virus from entering human cells, less light would indicate that the antibodies protected against infection, he added.

Luciferase, the same enzyme that gives fireflies their glow, is helping USF Health researchers track how much proxy COVID-virus invades human cells and which cells are most vulnerable.
Structural biology: A key to drug discovery
Unraveling the structure of viral proteins and identifying the receptors they use to enter cells can help guide discovery and design of potential antiviral treatments.
Yu Chen, PhD, is a USF Health associate professor of molecular medicine with a background in structural biology and biochemistry. Dr. Chen applies his expertise in structure-based drug design using advanced techniques — including X-ray crystallography and molecular docking — to help develop inhibitors (drug compounds) that target bacterial enzymes causing resistance to certain commonly prescribed antibiotics such as penicillin.
Now he’s turned his attention toward looking for new or existing drugs to stop SARS-CoV-2.

Yu Chen, PhD, an associate professor of molecular medicine who has expertise in structure-based drug design, has turned toward looking for new or existing drugs to stop SARS-CoV-2.
One way to do this would be to block the virus’s main protease, known as Mpro, an enzyme that cuts out proteins from a long strand that the virus produces when it invades a cell. Without it, the virus cannot replicate. Dr. Chen works with colleagues at the University of Arizona College of Pharmacy (Jun Wang, PhD) and the USF Department of Chemistry (James Leahy, PhD) on this project.
“Mpro represents a promising target for drug development against COVID-19 because of the enzyme’s essential role in viral replication and the absence of a similar protease in humans,” Dr. Chen said. Since people do not have the enzyme, drugs targeting this protein are less likely to cause side effects, he explained.
This winter, an international team of scientists shared their description of the complex crystal structure of Mpro and in April published their discovery of its inhibitors, a half-dozen leading drug candidates identified by targeting the viral enzyme. Taking advantage of the breakthrough, Dr. Chen and other scientists worldwide hope to add more candidates to the drug discovery pipeline soon.
Together with the scientists from University of Arizona, Dr. Chen has found that several known protease inhibitors, including an FDA-approved hepatitis C (HCV) drug boceprevir and an investigational veterinary antiviral drug GC376, showed potent inhibition of the viral protein, and were more active than the previously identified inhibitors. Dr. Chen and his doctoral student, Michael Sacco, have recently determined the first structure of GC376 bound by Mpro, and characterized the molecular interactions between the compound and the viral enzyme. Their paper describing these results will soon be published in the prestigious scientific journal Cell Research.

Generated by X-ray crystallograhy, this image depicts the overall structure of the COVID-19 virus’s main protease (Mpro), which plays a key role in viral replication. Dr. Chen and colleagues recently found two new protease inhibitors that offer promise in blocking the drug target. –Photo courtesy of Yu Chen.
Dr. Chen and colleagues are also looking for small molecules that can effectively stop the Mpro enzyme from working or last long enough in the body to kill the COVID-19 virus.
The researchers use the latest computer software to visualize and predict how different drug candidates (Mpro inhibitors) bind with the viral proteins. This 3D structural analysis of “binding hotspots” can help in designing and chemically modifying other types of protease inhibiting-drugs with improved activity against SARS-CoV-2, Dr. Chen said.
The most potent antiviral compounds would be tested in human respiratory cell cultures growing the virus. Only then can a drug candidate move to animal models, and, eventually, human trials.
Genomics: Linking genetic variations to outcomes
Why do some individuals get so ill from the COVID-19 virus, while others barely notice symptoms? Why do certain countries and populations have higher death rates than others? Age, underlying medical conditions, socioeconomic and environmental factors play a role – but genetic variation, both in the virus itself and the humans it invades, are likely part of the equation.
“This virus has swept across the world, and some differences in immune response, virulence and disease outcomes of people infected with SARS-CoV-2 could be due to various strains of the virus yet to be defined,” USF Health’s Dr. Liggett said.

Differences in immune response, virulence and disease outcomes of people infected with SARS-CoV-2 could be due to various strains of the virus not yet defined, Dr. Liggett says.
Sequencing all genes that make up the COVID-19 virus — not just certain sections of the virus’s genome — will be key to uncovering genetic changes that could make a difference in patient susceptibility and outcomes, Dr. Liggett said. More than a decade ago, a team led by Dr. Liggett sequenced for the first time all known genomes of the human rhinovirus, providing a framework for antiviral treatments or vaccine development for this common respiratory virus implicated in asthma flare-ups.
“All parts of a virus’s genome work together for its existence, reproduction and infectivity,” he said. “So, to sequence only one part would be like looking at just the spark plugs, instead of the whole engine, when your car is not running well.”
The data gathered so far indicates that SARS-CoV-2 mutates slowly in the population. Most people have only 10 or so genetic variations in the 30,0000 nucleotide viral genome compared to the reference standard, Dr. Liggett said. “This may be a good sign that antibodies developed from an infection, a vaccine, or derived from an infusion, will provide long-lived immunity. This lower level of mutations also allows us to track a viral strain, potentially knowing how a community became infected.”
Noting where genetic variation does not occur is also important, since this may represent a “soft-spot” in the virus’s genome that cannot tolerate change because it is so vital, he added. “That might offer a clue about where to target a vaccine or therapy.”
As for human genetic variations that might influence whether certain individuals or subgroups of patients with COVID-19 fare better or worse, Dr. Liggett says the scientific community understands many human genes responsible for mounting an immune defense against this SARS-CoV-2 virus, and other respiratory viruses.
“With enough samples and epidemiology, we may be able to identify patients at genetic risk for serious, life-threatening outcomes,” he said. “However, it will be extremely challenging to find those needles in this big haystack.”
Clinical Trials: Testing treatments that attack on several fronts
As clinicians cared for more patients, one thing became increasingly clear – COVID-19 is more than a respiratory disease that injures the lungs.
It can strike many cell types and organs throughout the body including the brain, heart, blood vessels and kidneys; destroy taste and smell; cause life-threatening blood clots; and trigger a dangerous inflammatory cytokine storm. People with weakened immune systems are more vulnerable to severe illness – including the elderly and those with heart or lung diseases, diabetes, obesity or other underlying medical conditions. Black and Latino populations are disproportionately more likely to die from the virus. And while children are largely spared, a rare inflammatory pediatric syndrome with cardiac complications has been associated with COVID-19.

USF Health, working with Tampa General Hospital, had been at the forefront of a wide range of COVID-19 clinical trials in the Tampa Bay region.
Physicians and scientists are exploring many possible treatments to increase survival and improve prognoses for critically ill patients. Some target the virus itself or human cellular pathways that the virus exploits to replicate. Others aim to prevent collateral inflammatory damage in the human host. A disease affecting so many parts of the body will need drugs, or combinations of drugs, to attack on several fronts, said USF Health infectious disease physician-scientist Dr. Kim.
In the Tampa Bay region, USF Health, working with Tampa General Hospital, is at the forefront of a wide range of COVID-19 clinical trials. Creating drugs from scratch can take years, so several trials are investigating medications already prescribed for other infectious or inflammatory diseases to determine their effectiveness against SARS-CoV-2. For instance, Dr. Kim is local lead investigator for a multisite randomized controlled trial testing the safety and effectiveness of sarilumab in blocking acute lung damage in hospitalized COVID-19 patients. Sarilumab, approved for treating rheumatoid arthritis, is a monoclonal antibody targeting the proinflammatory cytokine receptor interleukin 6. Another trial will evaluate the ability of nitazoxanide, originally developed as an antiparasitic drug for gastrointestinal infections, to prevent respiratory virus replication in health care workers.
Dr. Kim is also working with Tampa General’s laboratory to analyze and validate the reliability of commercial tests that test patient blood samples for antibodies, proteins that provide evidence of past COVID-19 infection and recovery.

Kami Kim, MD, director of the Division of Infectious Disease and International Medicine at USF Health, leads a study evaluating the accuracy of antibody testing.
The accuracy of the antibody testing – different from the nasopharynx swab or saliva tests used to diagnose a current active infections – is important because it can give health officials a clearer picture of how widely COVID-19 has spread in the community and the extent of asymptomatic cases. Based on past experience with other coronaviruses like SARS and MERS, a positive SARS-CoV-2 antibody test would typically indicate some level of immunity. Researchers like Dr. Kim want to confirm that and hope to define the concentration of antibodies needed to confer immunity as well as how long that immunity lasts.
(In late May, the Centers for Disease Control and Prevention released new guidelines cautioning that some antibody tests have high false positive rates, and more definitive data is needed before they can be used to make decisions about returning to work, school or other public places.)
“We need to know if people who have the antibodies are actually protected against another infection,” Dr. Kim said. “It’s not yet clear… but, preliminary data indicates that a fairly large proportion of those people who recover from COVID-19 infection will have what are the protective (neutralizing) antibodies.”
SARS-CoV-2 shares genetic and some clinical similarities with the first SARS virus (SARS-CoV) — which caused a smaller scale global outbreak and has not re-emerged since the last reported case in 2004. But the new coronavirus is both more highly contagious and more apt to spread asymptomatically.

Based on past experience with other coronaviruses like SARS and MERS, a positive SARS-CoV-2 antibody test would typically indicate some level of immunity. Scientists are working to figure out how much immunity and how long it lasts.
“It’s the thing that has kept all of us in public health and infectious diseases up at night – a completely new pathogen that explodes before we had a real chance to get a handle on what was happening,” Dr. Kim said. “We’re learning more as we go, but teamwork is essential. No one will be able to solve all the pieces of this pandemic puzzle by themselves,” she added.
It will take time for scientists to fully understand the COVID-19 virus and how genetics, the environment, medications, lifestyle and public health measures impact the course of the disease.
“COVID-19 has essentially shut down the entire world,” added Dr. Kim, who as a clinical infectious diseases fellow at the University of California San Francisco in the 1980s witnessed firsthand the devastating consequences of the domestic HIV/AIDS epidemic. “A lesson we need to learn is the importance of maintaining preventive public health infrastructures — not only in our local communities, but globally, so that we can efficiently combat any future pandemics.”





