The five-year, $4.9-million NIH project aims to help eliminate the world’s most geographically widespread type of malaria
With the support of a five-year, $4.86-million National Institutes of Health grant, Distinguished University/USF Health Professor John Adams, PhD, leads a team of international researchers focused on accelerating the discovery of a vaccine against Plasmodium vivax malaria, a major global health problem.
The new grant from the NIH’s National Institute of Allergy and Infectious Diseases (NIAID) recognizes that a vivax malaria vaccine is among the tools critically needed to meet the World Health Organization’s (WHO) ambitious goal of eliminating 90% of global malaria cases and associated deaths by 2030. Of the five species of Plasmodium parasites that infect humans (starting with the bite of an infected mosquito), Plasmodium vivax (P. vivax) is the most prevalent type of malaria outside Africa, mostly in Asia and Latin America. Over a third of the world’s population live at risk of infection from P. vivax malaria, according to the WHO.
“We’re working on identifying a few good vaccine targets from the mosquito-to-human transmission stage and figuring out how to package and deliver them in a way that induces a broadly protective immune response against all stages and strains of vivax malaria,” said Dr. Adams, a professor in the USF College of Public Health, who has dedicated his career to finding solutions for malaria. “The ultimate goal is getting a combination vaccine that will block infection, the disease, and transmission of the parasite into clinical trials.”
The institutions collaborating with USF Health on the vaccine development project are the University of Florida, Johns Hopkins University, Mahidol University, the Armed Forces Research Institute of Medical Science, the Myanmar Health Network Organization, and U.S. Naval Medical Research Unit 6.

John Adams, PhD, a professor in the USF College of Public Health, has dedicated his career to finding solutions for malaria, a leading global cause of illness and death. | Photo by Allison Long, USF Health Communications
In their race to create a viable vaccine for an ancient disease that has evolved ways to evade human immunity since the dawn of humanity, Dr. Adams and his co-investigators build on previous successes to create transformative technologies for malaria research. These include a laboratory method developed by Dr. Adams’ research group to radically improve how scientists can study liver-stage malaria in vitro, allowing better preclinical assessment of new vaccines and drugs.
Challenges posed by vivax malaria
While less deadly than Plasmodium falciparum malaria (the leading cause of malaria), vivax malaria is far from benign.
Endemic mostly in poor countries, it exacts an estimated economic burden of $1.4 to $4 billion a year, including health care costs and absences from school or work. Like P. falciparum, P. vivax symptoms include fever, chills, headaches and muscle aches, and the parasitic disease can be debilitating and cause severe anemia in infants.
Several challenges make vivax malaria transmission more difficult to control than malaria caused by P. falciparum, Dr. Adams said. These include difficulty detecting the P. vivax parasite with existing diagnostic tests; the parasite’s ability to survive in cooler climates, thus extending its geographic range; and a dormant liver-stage form of the parasite that can reactivate weeks, months or even years after initial infection, causing multiple relapses and more opportunities for transmission. In addition, Dr. Adams said, the only type of drug effective against the dormant liver-stage parasite, 8-aminoquinolines, may cause severe, potentially lethal blood loss in people deficient in glucose phosphate dehydrogenase (G6PD) and no simple test can detect this enzyme deficiency.
“Places where vivax malaria prevails lack good access to affordable health care and potential complications exist with drug treatment and compliance, so developing vaccines to help protect against disease makes a lot of sense,” Dr. Adams said.

Above and below: Mosquitoes used by Dr. Adams and his team to study malaria parasites are kept in secure containers in an insectary at USF. | Photos by Allison Long, USF Health Communications
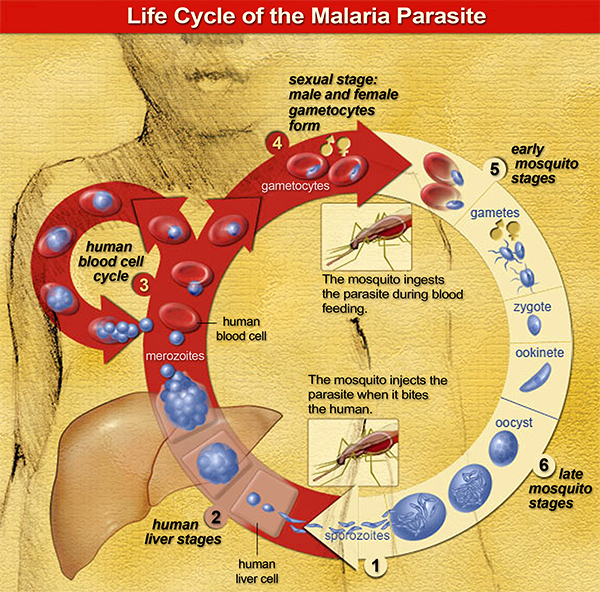
The genome of the malaria parasite is larger and more complex than those of bacteria or viruses. Add to that a complicated life cycle that occurs both in mosquitoes and humans (described in this animation), and malaria presents an enduring challenge for vaccine development.
Only a few vaccine candidates for P. vivax have advanced to small, initial clinical trials, Dr. Adams said. And the only approved P. falciparum vaccine (MosquirixTM), administered to young children in sub-Saharan Africa, showed only partial protection in field trials.
Identifying a vaccine with broad protective immunity
To increase the odds of a finding a vaccine offering broad, sustained protection against vivax malaria, the NIAID-funded global team led by Dr. Adams focuses on identifying the best targets to interfere with different life stages of the parasite. This approach, he said, can be combined with another key vaccine strategy that aims to block diverse strains (variants) of the same malaria parasite.
“A major barrier to vaccine effectiveness is the ability of the parasite to change its (structural) appearance as recognized by our immune system without compromising its ability to survive or infect a person,” Dr. Adams said. Over time molecular changes in antigens — proteins or parts of proteins on the surface of the invading pathogen — can allow the malaria parasite to evade a vaccine-induced immune response.
“We are witnessing the same phenomenon playing out right now with COVID-19 variant strains,” Dr. Adams added.

Microscopic image of an Anopheles stephensi mosquito, which can transmit both the vivax and falciparum malaria parasites. | Photo by Allison Long, USF Health Communications
A vaccine trains your immune system to produce antibodies (proteins that fight disease) just like it would if you were exposed to the infection naturally. Dr. Adams and colleagues identify exactly which cell surface molecules that neutralizing antibodies latch onto to inhibit the P. vivax parasite’s entry into and infection of the human host cell. To overcome the problem of malaria parasites evading vaccine immunity, they look for “functionally conserved” antigens; that is, specific parasite protein regions targeted by protective neutralizing antibodies that resist variation because they are essential to the pathogen’s function. The researchers use data on the quality of neutralizing antibodies collected from malaria patients in P. vivax-endemic Thailand to help determine optimal targets for preventing parasite development.
The team has already identified promising conserved vaccine targets for several life stages of the P. vivax parasite — those that block infection in human blood and liver, and in the mosquito itself. They continue to seek effective targets to attack parasites after they are injected by the mosquito but before they move to the liver.
“The trick with vaccines is not just the combination of (antigen) targets you assemble to elicit a good immune response, but the package used to deliver all the pieces of the vaccine,” Dr. Adams said.
The researchers plan to test delivery of their leading vaccine candidates using both messenger RNA and nanoparticle platforms. The Pfizer and Moderna vaccines for COVID-19 rolled out late last year were the first to successfully apply mRNA technology, which can be easily adapted to incorporate new and expanded vaccine targets.
“It’s an exciting time for vaccine development,” Dr. Adams said. “We’re hopeful that the remarkable scientific advances in vaccine technology made to combat COVID-19 will benefit other infectious diseases, including malaria.”