The USF Health Byrd Alzheimer’s Center contributed to a new international study in Nature Medicine suggesting there is reason to reevaluate the concept of “typical” Alzheimer’s disease. The study examined the largest and most diverse population in the world to date using tau-positron emission tomography scans (tau-PET scans), an advanced neuroimaging technique.
Amanda Smith, MD, professor of psychiatry and behavioral neurosciences and clinical research director at the Byrd Alzheimer’s Center, USF Health Morsani College of Medicine, was among the Alzheimer’s Disease Neuroimaging Initiative (ADNI) coauthors for the Nature Medicine paper. As one of more than 60 ADNI sites across the U.S. and Canada, the Byrd Alzheimer’s Center shares PET and MRI images, cognitive tests, blood biomarkers and other research data used by scientists worldwide to improve the understanding of Alzheimer’s disease.
Alzheimer’s disease is characterized by toxic accumulation of the protein tau (as well as abnormal amyloid protein deposits), leading to the death of nerve cells in the brain.
The recent study, led by researchers from McGill University and Lund University, delineates four distinct patterns (subtypes) of tau pathology in Alzheimer’s disease — each distinguished by where in the brain toxic tau deposits originate and spread. The researchers showed that over time each pattern of tau accumulation correlates to different clusters of symptoms with different prognoses for the affected individuals.
For the past 30 years, many researchers have described the development of tau pathology in Alzheimer’s using a single model, despite recurring cases that do not fit that model.
The current findings help explain why different patients may develop different symptoms, Dr. Smith said.
“In the clinic where we assess hundreds of patients with Alzheimer’s disease, we know that not everyone presents with the same symptoms. Many people present with typical short-term memory loss. Some can remember but exhibit very prominent language problems. Others may have visual difficulties that cause them to not see, or to misinterpret, what is front of them,” she said. “Although advanced Alzheimer’s tends to look the same, individuals don’t necessarily fit neatly into one category (of symptoms) earlier in the disease process.”
The recent tau-PET scan findings have implications for how disease progression is staged, and ultimately helping with the discovery of individualized treatments.
Byrd neuroscientists are working to develop both anti-amyloid and anti-tau antibodies – drugs to stop or delay Alzheimer’s disease, which yet has no disease-modifying therapies. In addition to more precisely detecting the early presence of disease and monitoring its progression, the latest neuroimaging techniques help researchers see whether their investigational drugs can remove the damaging Alzheimer’s-associated proteins from the brain.
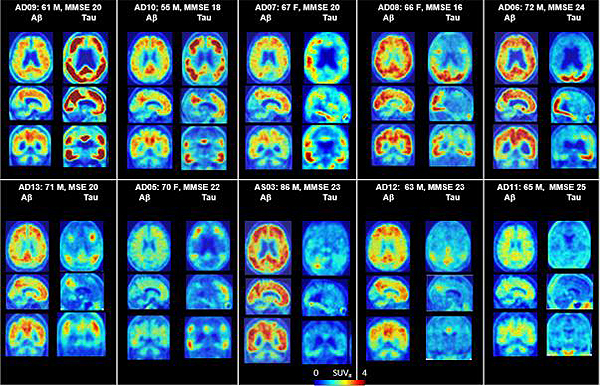
PET scans of the brains of Alzheimer’s patients, showing patterns of both amyloid and tau. CREDIT: Dean Wong, MD, PhD, Ayon Nandi, MS, and Hiroto Kuwabara, MD, PhD | Johns Hopkins Medicine
“The increasing degree of specificity provided by neuroimaging studies may advance our ability to accurately target treatments for individuals with abnormal tau in the brain – and that’s not just limited to Alzheimer’s disease,” Dr. Smith said. “While amyloid is unique to Alzheimer’s, toxic tau is found in other cognitive disorders, including certain frontotemporal dementias and chronic traumatic encephalopathy.” (CTE is brain degeneration linked to repeated head trauma, including concussions in athletes.)
Dr. Smith leads clinical trials at the USF Health Byrd Alzheimer’s Center that involve brain imaging of a wide range of older adults – from study participants with no symptoms (presymtomatic) or very minor memory difficulties, to those diagnosed with mild cognitive impairment or various stages of Alzheimer’s dementia.
Current trials include Alzheimer’s Disease Initiative 3, the AHEAD Study, and the Trial-Ready Cohort for the Prevention of Alzheimer’s Dementia (TRC-PAD). For information on these and other clinical studies, please visit health.usf.edu/medicine/byrd/clinical-trials, or call 813-974-4904.

