]]>
USF Health researchers applied CRISPR technology to study the very large human non-protein coding gene expressed only in placenta, stem cells and certain cancers
TAMPA, Fla (March 16, 2020) — The placenta, an organ which attaches to the lining of the uterus during pregnancy, supplies maternal oxygen and nutrients to the growing fetus. Abnormal formation and growth of the placenta is considered an underlying cause of various pregnancy complications such as miscarriages, stillbirth, preeclampsia and fetal growth restriction. Yet, much remains to be learned about molecular mechanisms regulating development of this blood-vessel rich organ so vital to the health of a pregnant woman and her developing fetus.

Hana Totary-Jain, PhD, an associate professor of molecular pharmacology and physiology in the USF Health Morsani College of Medicine, was senior author of the study published in Scientific Reports.
University of South Florida Health (USF Health) Morsani College of Medicine researchers recently discovered how a very large human non-protein coding gene regulates epithelial-to-mesenchymal transition (EMT) – a process that contributes to placental development during early pregnancy, but can also promote cancer progression.
During the first trimester, fetal-derived placental cells known as trophoblasts invade the maternal uterine lining and modify its blood vessels to allow oxygenated blood to flow from the mother to fetus. However, trophoblast invasion requires tight regulation of EMT. If inadequate, trophoblast invasion is too shallow to adequately remodel the maternal blood vessels, and adverse pregnancy outcomes can occur. Conversely, excess EMT can cause exaggerated trophoblast invasion through the uterine wall leading to placenta accreta, a condition that can cause hemorrhage and often requires hysterectomy at delivery.
The USF Health researchers used a powerful genome editing technology called CRISPR (shorthand for “CRISPR-dCas9) to activate all of the chromosome 19 microRNA cluster (known as C19MC), so they could study the gene’s function in early pregnancy. C19MC — one of the largest microRNA gene clusters in the human genome — is normally turned off but becomes expressed only in the placenta, embryonic stem cells and certain cancers.

Dr. Totary-Jain discusses the molecular aspects of placenta development and pregnancy complications with research collaborator Umit Kayisli, PhD, a professor of obstetrics and gynecology at USF Health.
In their cell model study, published Feb. 20 in Scientific Reports, a Nature research journal, the USF Health team showed that robust activation of C19MC inhibited EMT gene expression, which has been shown to reduce trophoblast invasion.
But when trophoblast-like cells were exposed to hypoxia – a lack of oxygen similar to that occurring in early placental development — C19MC expression was significantly reduced, the researchers found. The loss of C19MC function causes differentiation of trophoblasts from stem-like epithelial cells into mesenchymal-like cells that can migrate and invade much like metastatic tumors. This EMT process helps explain trophoblast invasion and early placental formation.
“We were the first to use CRISPR to efficiently activate the entire gene, not just a few regions of this huge gene, in human cell lines,†said the paper’s senior author Hana Totary-Jain, PhD, an associate professor in the Department of Molecular Pharmacology and Physiology, USF Health Morsani College of Medicine. “Our study indicates C19MC plays a key role in regulating many genes important in early implantation and placental development and function. The regulation of these genes is critical for proper fetal growth.â€

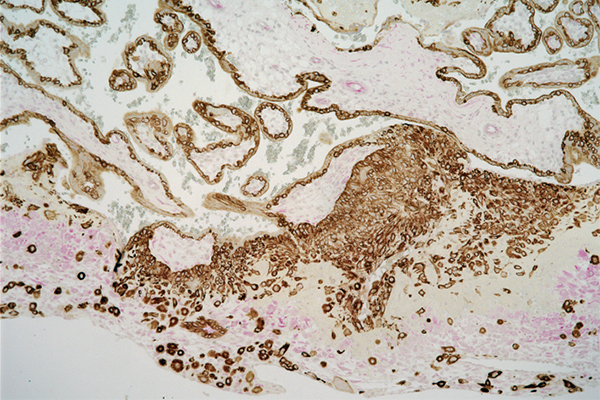
Above: Chromosome 19 microRNA cluster (stained purple) expressed in first-trimester placenta. Below: In preparation for pregnancy, fetal trophoblast cells (brown) from which the placenta arises invade maternal decidual cells (pink) in the uterus lining. | Images courtesy of Hana Totary-Jain, originally published in Scientific Reports: doi.org/10.1038/s41598-020-59812-8
“You need EMT, but at some point the process needs to cease to prevent adverse pregnancy outcomes,†Dr. Totary-Jain said. “You really need a balance between not enough invasion and too much invasion, and C19MC is important in maintaining that balance.â€
Dr. Totary-Jain and others in her department collaborated with colleagues in the medical college’s Department of Obstetrics and Gynecology on the project.
“The USF Health study offers new insight into how trophoblasts interact with the maternal uterine environment to become more invasive or less invasive in the formation of the placenta,†said coauthor Umit Kayisli, PhD, a USF Health professor of Obstetrics and Gynecology. “More research on microRNA expression and how it inhibits EMT may help us better understand the causes and potential prevention of preeclampsia and fetal growth restriction, which account for 5-to-10 percent of all pregnancy complications as well as spontaneous preterm births.â€
Investigating the effects of altered C19MC expression on cell differentiation and trophoblast invasion has implications not only for a better understanding of normal and abnormal placental development, but also for cancer and stem cell research, Dr. Totary-Jain added.
Photos by Freddie Coleman, USF Health Communications and Marketing
]]>
]]>
Carrie Ryan got the text message while she was in her Introduction to Clerkship class at the USF Health Morsani College of Medicine: she was a potential match to someone needing a bone marrow transplant.
Ryan, a third-year medical student at USF Health, had added her profile to the National Marrow Donor Program several years prior while she worked in Washington, DC. With a simple inner-cheek swab, her genetic information was added to the national registry of millions of people willing to offer their bone marrow to others in need of life-saving stem cells.
“I had been on the registry for eight years or so,†Ryan said. “So I knew from the day I registered that I could be contacted at any given moment.â€
After the text message, Ryan’s next steps were to provide a blood sample to confirm through HLA typing that she would match with the recipient, answer a detailed questionnaire about her current health status and exposure to infectious diseases such as Zika virus, among other questions, and undergo a physical examination, EKG and a chest X-ray.
During this time, Ryan also found out a little more about her recipient.
“She is 2 years old and has leukemia,†Ryan said. “Talk about an incentive to help.â€
A week prior to her surgery, Ryan underwent filgastrim (Neupogen®) injections to stimulate her body into producing more bone marrow. She then flew to Washington, DC, where she underwent peripheral blood stem cell collection. The donation is through apheresis, a process similar to the donation of platelets, which took about five hours and she was able to return to Tampa the following day.
Although she hasn’t met her recipient, Ryan said she was told the toddler is doing well.
November is National Marrow Awareness Month and Ryan said she’s eager to remind others how easy it is to be included in the national registry and how impactful it can be to the thousands who are waiting for a match.
“Only about 30 percent of family members are matches so it’s important that we all add to the registry,†she said. “It’s super easy and super rewarding.â€
There is a national need for bone marrow donors, especially for minority groups. You can register through a local registration drive, or you can sign up on BeTheMatch.org.
//www.youtube.com/watch?v=Hq_pfozYqtM
Photos and video by Ryan Noone, USF Health Office of Communications
]]>
]]>
A USF preclinical study shows transplanted human bone marrow stem cells preferentially migrate to the spleen, reducing systemic inflammation of later-stage stroke
Tampa, FL (Sept. 15, 2015) — Stroke injures the brain, but a new University of South Florida study indicates an abdominal organ that plays a vital role in immune function, the spleen, may be a target for treating stroke-induced chronic inflammation leading to further brain cell death.
Neuroscientists at the USF Center of Excellence for Aging and Brain Repair found that human bone marrow stem cells intravenously administered to post-stroke rats preferentially migrated to the spleen and reduced the inflammatory-plagued secondary cell death associated with stroke progression in the brain. The study is reported in the September 2015 issue of the American Heart Association journal Stroke.

Cesario Borlongan, PhD, the study’s principal investigator, is a pioneer in stem cell therapy research for stroke. He directs the USF Center of Excellence for Aging and Brain Repair.
The USF study helps resolve a perplexing observation by many scientists evaluating the effects of stem cell therapies: Functional recovery occurs in experimental models of neurological disorders, including stroke, despite little or mediocre survival of transplanted stem cells within the injured brain.
“Our findings suggest that even if stem cells do not enter the brain or survive there, as long as the transplanted cells survive in the spleen the anti-inflammatory effects they promote may be sufficient enough to therapeutically benefit the stroke brain,†said principal investigator Cesario Borlongan, PhD, professor and director of the USF Center of Excellence for Aging and Brain Repair.
Stroke is a leading cause of death and the number one cause of chronic disability in the United States, yet treatment options are limited. Stem cell therapy has emerged as a potential treatment for ischemic stroke, but most preclinical studies have looked at the effects of stem cells transplanted during acute stroke – one hour to 3 days after stroke onset.
Following acute stroke, an initial brain attack caused by lack of blood flow, the blood-brain barrier is breeched, allowing the infiltration of inflammatory molecules that trigger secondary brain cell death in the weeks and months that follow. This acerbated inflammation is the hallmark of chronic stroke.
The USF researchers intravenously administered human bone marrow stem cells to rats 60 days following stroke onset – the chronic stage. The transplanted stem cells were attracted predominantly to the spleen; the researchers found 30-fold more stem cells survived in this peripheral organ than in the brain. Once in the spleen, the stem cells dampened an inflammatory signal (tumor necrosis factor) activated immediately after stroke and prevented the migration from spleen to the compromised brain of harmful macrophages that stimulate inflammation.
This reduced systemic inflammation correlated with significant decreases in the size of lesions caused by acute stroke in the striatum—a portion of the brain controlling movement. There was a trend toward prevention of additional neuron loss in the portion of the brain affecting memory and thinking.
“In the chronic stage of stroke, macrophages are like fuel to the fire of inflammation,†Dr. Borlongan said. “So if we can find a way to effectively block the fuel with stem cells, then we may prevent the spread of damage in the brain and ameliorate the disabling symptoms many stroke patients live with.â€
The USF researchers next plan to test whether transplanting human bone marrow stem cells directly into the spleen will lead to behavioral recovery in post-stroke rats.
The one drug approved for emergency treatment of stroke, the clot-busting drug tPA, must be administered less than 4.5 hours after onset of ischemic stroke, and benefits only 3 to 4 percent of patients, Dr. Borlongan said. While more study is needed, evidence from USF and other groups thus far indicates stem cells may help provide a more effective treatment for stroke over a wider timeframe.
“Stem cells are not a magic bullet, but a combination of stem cells and other anti-inflammatory agents may lead to the optimal therapeutic benefit for stroke patients,†he said.
Lead study author Sandra Acosta, PhD, a postdoctoral fellow in the USF Department of Neurosurgery and Brain Repair, said targeting the spleen with stem cells or the anti-inflammatory molecules they secrete offers hope for treating chronic neurodegenerative diseases like stroke at later stages.
“We’ve shown (in an animal model) that it’s possible to stop disease progression 60 days after the initial stroke injury, when chronic inflammation in the brain was widespread,†she said. “If that can be replicated in humans, it will be powerful.â€
The USF study was supported by grants from the National Institute of Neurological Disorders and Stroke and the James and Esther King Biomedical Research Foundation.
Article citation:
Sandra A. Acosta; Naoki Tajira; Jaclyn Hoover; Yuji Kaneko; and Cesar Borlongan, “Intravenous Bone Marrow Stem Cell Grafts Preferentially Migrate to Spleen and Abrogate Chronic Inflammation in Stroke,†Stroke, September 2015; DOI: 10.1161/STROKEAHA.115.009854.
                                                                                                      About USF Health
USF Health’s mission is to envision and implement the future of health. It is the partnership of the USF Health Morsani College of Medicine, the College of Nursing, the College of Public Health, the College of Pharmacy, the School of Physical Therapy and Rehabilitation Sciences, and the USF Physician’s Group. The University of South Florida is a Top 50 research university in total research expenditures among both public and private institutions nationwide, according to the National Science Foundation. For more information, visit www.health.usf.edu
Media contact:
Anne DeLotto Baier, USF Health Communications
abaier@health.usf.edu or (813) 974-3303
Photos by Sandra Roa, USF Health Communications and Marketing
]]>
]]>
By Saundra Amrhein
As a graduate student David Birk, PhD, developed an interest in connective tissues and the cells that produced these molecular building blocks. He became fascinated by the question of how the self-assembly of collagen fibrils and fibers outside of cells was regulated as well as by how these extracellular matrix components (ECM) influenced the cells residing in them.

Dr. David Birk’s ongoing work provides the biological foundation needed for understanding connective tissue diseases and potential therapeutic interventions.
Thirty-five years later, Dr. David Birk’s decades of research and ongoing work with collagens and a family of proteins known as small leucine rich proteoglycans have led to a groundbreaking understanding of the extracellular matrix or framework involved in the development of connective tissue. His work on the regulation of ECM assembly has major implications for current and future treatments of connective tissue disorders ranging from poor wound healing and scarring, to progressive fibrotic diseases, to corneal blindness to congenital diseases such as Ehlers-Danlos syndrome (EDS).
“There’s probably not a system in the body not affected by connective tissue,†said Dr. Birk, a professor of molecular pharmacology and physiology at the University of South Florida.
Dr. Birk’s work influences not only the understanding of, and treatments for connective tissue disorders, but also future treatments for common tendon and ligament injuries, such as the tearing of the anterior cruciate ligament, or the ACL.
All told, more than 200 types of connective tissue disorders impact millions of people worldwide. The connective tissue – which is the glue or cement holding the body together, from ligaments to tendons, to skin, corneal stroma, cartilage and bone – can be altered in numerous ways. This results from injuries and scarring. And many stem from genetic mutations inherited from parents. This includes osteogenesis imperfecta (where bones break easily); Marfan syndrome (often affecting the heart, blood vessels, lungs); and Ehlers-Danlos syndrome, or EDS (marked by loose joints, stretchy skin, small blood vessels and abnormal wound healing).

Using electron microscopy, Dr. Birk has mapped out the process of collagen fibril assembly and developed a model for regulation of matrix assembly. He worked with human geneticists to create mouse models for diseases of human connective tissues.
Last year, Dr. Birk – who came to USF in 2007 from Thomas Jefferson University in Philadelphia – was the principal investigator on a study that examined the roles of tendon-proper and peritenon-derived progenitor cells of the tendon in order to understand their unique capabilities in response to an injury. Using an in vitro model, his team found that the tendon-proper progenitors showed greater levels of tendon gene markers and matrix assembly genes for rebuilding or generating new functional tendon tissue in comparison to the peritenon-derived progenitor cells.  However, the latter also showed great potential, as well, when stimulated with growth factors. His team’s findings highlighted the “synergistic†potential of both types of cell populations that could be plugged into engineering models for restoring damaged tendons.
“My philosophy has always been if you understand the process of development, this can serve as a paradigm for regeneration,†Dr. Birk said. (To read the research, see: Stem Cell Research & Therapy 2014; 5(4): 86.)
Showing just that was another recent study in which Dr. Birk was involved and that may one day impact millions of people with corneal scarring and vision loss. Building upon Dr. Birk’s expertise in analysis of connective tissue structure and function, he worked with Dr. James Funderburgh and his group at the University of Pittsburgh who used human ocular stem cells derived from the corneal limbus in scarred and injured mouse corneas. Not only did the stem cells reestablish normal connective tissue architecture necessary for transparency at the scarred site, but healing in nearby tissue was promoted as well. This, according to the Pittsburgh group, suggested that the cells were not just replacing lost tissue, but were promoting regeneration.
The implications could one day be enormous for hundreds of millions of people suffering from and blinded by corneal infections, burns and other types of trauma and scarring to the cornea. It could possibly lead to the growth and use of stem cells from patients’ uninjured eye to heal the other – reducing the need for corneal transplants, which carry high risks of rejections. (See the full research in Science Translational Medicine, 10 Dec. 2014, Vol. 6, Issue 266, p. 266ra172.)
“Repairing anything with stem cells is a hot topic,†Dr. Birk said. “But I think what we have to recognize with stem cells, you can get them to differentiate along different paths, but if you want them to regenerate tissue structure and function, we need to define the context.â€
That context, or the study and knowledge of how tissues develop in the first place, has been at the heart of Dr. Birk’s career.
Among his accomplishments, he is considered a “pioneer†in understanding the role of the ECM in tissue development and other biological processes, said Louis J. Soslowsky, who is the Fairhill Professor of orthopaedic surgery; professor of bioengineering; founding director for the Penn Center for Musculoskeletal Disorders; and director of the McKay Orthopaedic Research Laboratory at the University of Pennsylvania.
Specifically, the model systems that Dr. Birk developed have provided “paradigm-shifting†research for the field, Dr. Soslowsky said.
“For example, his work crossing multiple tissues including tendon, ligament and cornea has shown that even small constituents – small by percentage of material – can play dramatically important regulatory roles in tissue development, function, aging and response to injury,†Dr. Soslowsky said. “His work is considered true ‘foundation work’ upon which he and others continue to build.â€
That “regulatory role†of a protein-like collagen was one of his major discoveries. When Dr. Birk started his research back in the 1970s and 1980s, it was understood that collagen helped assemble or form the structure of connective tissue, the glue or cement holding the body together. What was not known – and what Dr. Birk intuited from his research – was that collagen was instructional as well as structural. In other words, collagen was not just the cement holding the building of our bodies together. It also played the roles of architect and construction foreman.
“What’s been the biggest change in the past 30 years is that we’re not talking just about structural components, we’re talking about dynamic tissues,†Dr. Birk said.
Using electron microscopy, Dr. Birk proceeded to map out the process of collagen fibril assembly and developed a model for regulation of matrix assembly. He worked with human geneticists like Dr. Richard Wenstrup to create mouse models of diseases of human connective tissues.
In doing so, Dr. Birk found the essential instructional role of various collagens and the diseases that result not only in their absence or mutation but also from changes in collagens relative to other collagens in temporal or developmental stages of an embryo.
For instance, Dr. Birk and his team found that the absence of collagen V in development is lethal and reduced expression or mutation of collagen V results in the classic Ehlers-Danlos syndrome. Certain combinations revealed the variety of ways disorders can express themselves. Dr. Birk and his fellow researchers found that the absence of collagen XI with a reduction in collagen V were associated with the most severe fibril phenotype. Dr. Birk’s more recent studies examined how a mutation in the collagen XII gene creates skeletal abnormalities. He has also studied the role of key ECM regulatory molecules like decorin in the matrix assembly of tissue, from tendons to the cornea.
The animal models created by Dr. Birk and his researchers have become crucial tools used by other specialists to develop therapeutic interventions for patients with EDS and joint laxity. His discoveries have informed everything from genetic counseling to the diagnosis of mysterious multiple fractures in children seen in emergency departments.
Essential to this work and the advancements made in the field was Dr. Birk’s talent in building research collaborations across disciplines.
“In collaboration with myself and others, David has used that mouse model to really understand how human extracellular connective tissue develops,†said Dr. Wenstrup, who is currently the chief medical officer at Myriad Genetic Laboratories in Salt Lake City and who has collaborated with Dr. Birk from the 1990s to the present.
“Some collaborations work well and some don’t. It has a lot to do with the personality and the generosity of the individual collaborators, and working with David is such that he makes it desirable for different types of people with different types of skills to work together,†Dr. Wenstrup said. “He is an excellent research partner.â€
For his part, Dr. Birk feels confident that though there is no known cure for many of the connective tissue disorders he studies, great strides have been made in education, awareness and treatment based on the foundational work carried out by his team and other researchers through the years.
“I think we are making progress,†Dr. Birk said. “My interest is in providing a biological foundation necessary for understanding connective tissue diseases and potential therapeutic interventions, and that hopefully will provide a springboard for others with more clinical interests to pursue.â€
It’s a matter, he said, of continuing the research and integrating it with research conducted in clinical settings and in other disciplines.
“Looking back 30 plus years, we have made significant progress, the field has developed and the questions have changed,†he said. “We don’t understand the whole process, but a solid foundation is in place.â€

Dr. Birk and his research team collaborate nationally with other scientists in various disciplines — a key to advancement in the field.
Photos by Eric Younghans, USF Health Communications and Marketing
]]>
]]>
University of South Florida researchers have suggested a new view of how stem cells may help repair the brain following trauma. In a series of preclinical experiments, they report that transplanted cells appear to build a “biobridge†that links an uninjured brain site where new neural stem cells are born with the damaged region of the brain.
Their findings were recently reported online in the peer-reviewed journal PLOS ONE.
“The transplanted stem cells serve as migratory cues for the brain’s own neurogenic cells, guiding the exodus of these newly formed host cells from their neurogenic niche towards the injured brain tissue,â€Â said principal investigator Cesar Borlongan, PhD, professor and director of the USF Center for Aging and Brain Repair.

A team led by Cesar Borlongan, director of the University of South Florida Center for Aging and Brain Repair, offers a new concept for how transplanted stem cells help prod the brain’s own repair mechanism following traumatic brain injury.
Based in part on the data reported by the USF researchers in this preclinical study, the U.S. Food and Drug Administration recently approved a limited clinical trial to transplant SanBio Inc’s SB632 cells (an adult stem cell therapy) in patients with traumatic brain injury.
Stem cells are undifferentiated, or blank, cells with the potential to give rise to many different cell types that carry out different functions. While the stem cells in adult bone marrow or umbilical cord blood tend to develop into the cells that make up the organ system from which they originated, these multipotent stem cells can be manipulated to take on the characteristics of neural cells.
To date, there have been two widely-held views on how stem cells may work to provide potential treatments for brain damage caused by injury or neurodegenerative disorders.  One school of thought is that stem cells implanted into the brain directly replace dead or dying cells. The other, more recent view is that transplanted stem cells secrete growth factors that indirectly rescue the injured tissue.
The USF study presents evidence for a third concept of stem-cell mediated brain repair.
The researchers randomly assigned rats with traumatic brain injury and confirmed neurological impairment to one of two groups. One group received transplants of bone marrow-derived stem cells (SB632 cells) into the region of the brain affected by traumatic injury. The other (control group) received a sham procedure in which solution alone was infused into the brain with no implantation of stem cells.
At one and three months post-TBI, the rats receiving stem cell transplants showed significantly better motor and neurological function and reduced brain tissue damage compared to rats receiving no stem cells. These robust improvements were observed even though survival of the transplanted cells was modest and diminished over time.
The researchers then conducted a series of experiments to examine the host brain tissue.
At three months post-traumatic brain injury, the brains of transplanted rats showed massive cell proliferation and differentiation of stem cells into neuron-like cells in the area of injury, the researchers found. This was accompanied by a solid stream of stem cells migrating from the brain’s uninjured subventricular zone — a region where many new stem cells are formed – to the brain’s site of injury.
In contrast, the rats receiving solution alone showed limited proliferation and neural-commitment of stem cells, with only scattered migration to the site of brain injury and virtually no expression of newly formed cells in the subventricular zone. Without the addition of transplanted stem cells, the brain’s self-repair process appeared insufficient to mount a defense against the cascade of traumatic brain injury-induced cell death.
The researchers conclude that the transplanted stem cells create a neurovascular matrix that bridges the long-distance gap between the region in the brain where host neural stem cells arise and the site of injury. This pathway, or “biobridge,†ferries the newly emerging host cells to the specific place in the brain in need of repair, helping promote functional recovery from traumatic brain injury.
Article citation:
“Stem Cell Recruitment of Newly Formed Host Cells via a Successful Seduction? Filling the Gap between Neurogenic Niche and Injured Brain Site;â€Â Naoki Tajiri, Yuji Kaneko, Kazutaka Shinozuka, Hiroto Ishikawa, Ernest Yankee, Michael McGrogan, Casey Case, and Cesar V. Borlongan; PLOS ONE 8(9): e74857.  Published Sept. 4, 2013.
]]>
]]>
Tampa, FL (June 28, 2013) — A cardiac hormone signaling receptor abundantly expressed both in inflamed tissues and cancers appears to recruit stem cells that form the blood vessels needed to feed tumor growth, reports a new study by scientists at the University of South Florida Nanomedicine Research Center.Â
The research may lead to the development of new drugs or delivery systems to treat cancer by blocking this receptor, known as natriuretic peptide receptor A (NPRA).
The findings appeared online recently in the journal Stem Cells.
“Our results show that NRPA signaling by cancer cells produces some molecular factors that attract stem cells, which in turn form blood vessels that provide oxygen and nutrients to the tumor,†said the study’s principal investigator Subhra Mohapatra, PhD, associate professor in the Department of Molecular Medicine. “We showed that if the NPRA signal is blocked, so is the angiogenesis and, if the tumor’s blood supply is cut off it will die.â€Â

Subhra Mohapatra, PhD
Using both cultured cells and a mouse model, Dr. Mohapatra and her team modeled interactions to study the association between gene mutations and exposure to an inflammatory tissue microenvironment.
The researchers demonstrated that cardiac hormone NRPA played a key role in the link between inflammation and the development of cancer-causing tumors. Mice lacking NPRA signaling failed to induce tumors. However, co-implanting tumor cells with mesenchymal stem cells, which can turn into cells lining the inner walls of blood vessels, promoted the sprouting of blood vessels (angiogenesis) needed to promote tumor growth in NPRA- deficient mice, the researchers found. Furthermore, they showed that NRPA signaling appears to regulate key inflammatory cytokines involved in attracting these stem cells to tumor cells.
Dr. Mohapatra’s laboratory is testing an innovative drug delivery system using special nanoparticles to specifically target cancers cells like a guided missile, while sparing healthy cells. The treatment is intended to deliver a package of molecules that interferes with the cardiac hormone receptor’s ability to signal.
Dr. Mohapatra collaborated with Shyam Mohapatra, PhD, and Srinivas Nagaraj, PhD, both faculty members in the Nanomedicine Research Center and Department of Internal Medicine, on genetic and immunological aspects of the study.
The study was supported by the National Institutes of Health and a Florida Biomedical Research Grant.
-USF Health-
 USF Health’s mission is to envision and implement the future of health. It is the partnership of the USF Health Morsani College of Medicine, the College of Nursing, the College of Public Health, the College of Pharmacy, the School of Biomedical Sciences and the School of Physical Therapy and Rehabilitation Sciences; and the USF Physician’s Group. The University of South Florida is a global research university ranked 50th in the nation by the National Science Foundation for both federal and total research expenditures among all U.S. universities. For more information, visit www.health.usf.edu
Media contact:
Anne DeLotto Baier, USF Health Communications
abaier@health.usf.edu, or (813) 974-3303
]]>