Coronavirus Background
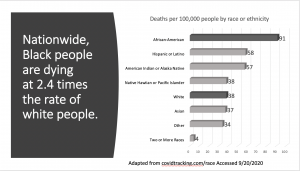
Greater than 198,000 Americans have died as a result of being infected by the novel Coronavirus (SARS-CoV-2) as of late September 2020. The first confirmed case in the United States occurred on January 20, 2020 ( N Engl J Med 2020; 382:929-936). According to the University of Washington Institute for Health Metrics and Evaluation (IHME), the projected number of Americans that may die from Coronavirus disease (COVID-19) by November 1, 2020 is greater than 229,000 (covid19.healthdata.org Accessed Sep 20, 2020). Unfortunately, the IHME model has proven to be fairly accurate during the duration of the national pandemic. As for the racial/ethnic mortality demographics of COVID-19, the largest percentage of the cases have been people of color, and in particular African Americans. (Yancy CW. JAMA. 2020;323(19):1891–1892; Journal of National Black Nurses Association. 31(1):1-12, 2020 Jul). From information posted on covidtracker.com (a link connected to CDC.gov), Blacks are 2.4 times more likely to die than Whites; Latinos and Native Indians are dying at 1.5 times the rate (See Image 1). This disproportionate effect on racial and ethnic American populations further spotlights the stench of health inequities that have befallen our country for decades, if not centuries (Smedley BD, Stith AY, Nelson AR, eds. Unequal Treatment: Confronting Racial and Ethnic Disparities in Health Care. : National Academies Press (US); 2003). In the midst of the current SARS-CoV-2 pandemic, this is a moment that now demands that the entire healthcare system – (patients, healthcare providers and institutions, public officials, and insurers) – all implement actions to drastically slow the death rate and the potentially lasting deleterious health effects of this virus.
During the past several months, a focus on vaccine development to combat the SARS-CoV-2 pandemic has reached a feverish pitch. The United States’ enhanced SARS-CoV-2 vaccine creation initiative, known as Operation Warp Speed, is focused on public-private partnerships with biotechnology pharmaceutical companies for COVID-19 vaccine development. As the name implies, the goal of this initiative is very rapid vaccine development. Commonly, new vaccine development can take anywhere from 5 – 10 years; the effort here is to develop a COVID-19 vaccine in approximately 9 months. While this seems quite expedient, much of this work actually began 15 years earlier during the original SARS-CoV outbreak in 2005. SARS-CoV (2005) was highly lethal, but not nearly as infectious (WHO Consensus document – (SARS) – 2003). While the SARS-CoV (2005) vaccine development initiatives greatly slowed during the past 15 years, the scientific platforms that were developed helped launch the current world-wide race to create a vaccine. When we add in predictive analytics, supercomputing, and advanced manufacturing practices, it becomes understandable how this may be achieved. It also becomes quite apparent why there may be heightened skepticism by the American general public concerning the safety of a vaccine developed this quickly. This skepticism is also quite elevated in the communities of color. Many individuals may ask themselves “Do I risk receiving a vaccine, or risk catching the virus?” With the deadly potential for COVID-19, or even the risk of long-term bodily injury by this novel Coronavirus, I implore everyone to strongly consider receiving an eventually approved vaccine that has undergone the appropriate FDA approval process. Here are my reasons.
Updates on The Current Coronavirus Research Clinical Trials
There are SARS-CoV-2 vaccine clinical trials emerging across the United States, and the globe. To date, no deaths directly attributed to the vaccine have been documented in the current clinical trials; there have been mostly mild to modest adverse effects documented for patients during the clinical trials (pain at injection site, elevated temperature, etc). These conditions were effectively managed with acetaminophen, and the duration of symptoms were short. More serious adverse effects have been noted in several phases of the trials; the numbers of these more serious effects have been very low when compared to the number of people that have received the test vaccinations worldwide. Continual reviews of possible adverse effects for all companies administering vaccine in clinical studies are conducted by their respective independent Data and Safety Monitoring Boards (DSMB) to determine if these challenges were due to the vaccine. DSMBs work independent of the companies to avoid conflicts of interest with pharmaceutical product testing during clinical trials. With tens of thousands SARS-CoV-2 vaccine doses having been given worldwide thus far in clinical trials, there is growing observational evidence that the vaccines are at least not producing widespread serious adverse effects. Now, more clinical trial research is needed to confirm the safety profiles of all of the various types of SARS-CoV-2 vaccines being developed, while simultaneously producing evidence that they will protect the user from developing COVID (Coronavirus disease).
Taking a Chance to Live – Time to Engage in the Solutions
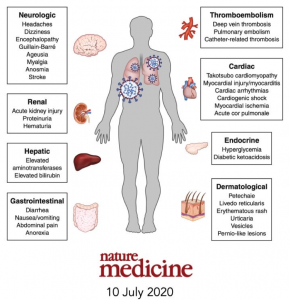
Early in the onset of the COVID-19 pandemic, progressive respiratory failure was the most known and recognizable clinical manifestation of COVID-19. Other very noticeable bodily effects began to emerge as the virus continued to spread throughout the globe. Cardiac disorders (mycarditis- inflammation in the heart; blood clots; heart attacks) have surfaced; neurological disorders (loss of smell and taste; mood disorders; headaches and dizziness); clotting disorders (blood clot development in the lungs and brain; small vessel clots); and gastrointestinal disorders (diarrhea, abdominal pain; nausea/ vomiting) among other disorders. (See Image 2) As the image shows, COVID-19 is far more than “just” a respiratory disease, and can produce very serious illness, bodily damage, and potential death of the infected individual.
Given that the beginning of SARS-CoV-2 has primarily occurred over the past seven months, scientists and clinician researchers have not had enough time to follow patients to see if these medical conditions will completely resolve in all patients. Persons infected by SARS-CoV-2 that have additional chronic health conditions (high blood pressure, diabetes, obesity, etc.) are at significant risk to experience the most severe health problems. Because of significant disparities in healthcare, including lack of access to quality healthcare and poorer social determinants of health (factors include: education, food access, neighborhood environment, socioeconomic status, etc.), African-Americans, Latinos, and American Indians (Image 1) have been most negatively affected (See Coronavirus And Health Equity- Again This (Too) Is Predictable). The potential for death following SARS-CoV-2 infection is elevated in these racial/ ethnic groups, despite the expanding use of therapeutic treatments (medications). And with the continued re-opening of the American economy, there is no reason to expect that infection rates will go down in our communities of color. More must be done to protect the most vulnerable among us.
I am urging individuals from Black/African-American, Hispanic/Latino, Native American, Asian Indian, and any other identified communities of color to participate in the clinical trials for SARS-CoV-2 vaccines. Before widespread distribution of any vaccine can be achieved, we must first gather credible data and information about the safety and efficacy of these vaccines. This can only be achieved through clinical research. While mistrust of clinical research in all of these communities has valid historical reason to exist, we must actively participate in the process to transparently gather and distribute all of the safety and efficacy data that is owed to every individual in all communities that seek to have such information (Scharff DP et al, More than Tuskegee: understanding mistrust about research participation. J Health Care Poor Underserved. 2010;21(3):879-897). Clinical researchers such as myself must be ever-present to watch and protect people from all communities. We must be available to help the restoration of trust in not only the clinical trial process, but in the healthcare system overall. Trust the Process, But Definitely Verify All Actions with Accountability and Transparency!
We are all faced with a foe in SARS-CoV-2 that has already proven to be deadly, and may leave the afflicted with long-term health ailments. As I mentioned in a recent community webinar, the most fundamental task we must attempt to accomplish each day is to stay alive. To live as productive a life as possible. And to be present for our children and loved ones as they proceed into a world that is changing more rapidly than our ability to keep up with it. This Coronavirus does not know color or ethnicity; it doesn’t know political affiliation; it doesn’t know rich or poor. It only knows its eventual victim as being a human being. We owe it to ourselves to fight back against it. Please Join Us in the battle against this virus. (See WE-CARE for more information in Florida)
Part II of Coronavirus Vaccines – “Trust, but Definitely Verify!” will follow shortly. There I will discuss the different types of Coronavirus vaccines presently being developed and studied, along with the necessity to hold all companies and our government accountable for equitable distribution of the vaccine to all communities once they are proven worthy for human application. Stay Tuned, and Stay Safe!!
Image 1. (Adapted from covidtracking.com/race – Accessed 9/20/2020)
Image 2 (Adapted from the journal Nature Medicine)