Greater than 237,000 Americans have died as a result of being infected by the novel Coronavirus (SARS-CoV-2) as of early November 2020. According to projections from the Institute for Healthcare Metrics (IHME -(covid19.healthdata.org Accessed November 6, 2020) an estimated 330,000 Americans, cumulatively, may die by January 1, 2020. There have been recent national surges in SARS-CoV-2 cases during the month October 2020. Deeply troubling is the daily diagnosed SARS-CoV-2 cases in early November topping 100,000 cases per day; it does not seem improbable that we may exceed 200,000 cases per day by December 1, 2020. As we have witnessed during the duration of this pandemic in our country, increased mortality rates typically lag directly behind increased infection rates. Now that COVID-19 cases are regularly exceeding 100,000 cases per day, healthcare workers will be expected to work at an extraordinarily challenging level to attempt to prevent a repeat of the pandemic mortality rates that occurred earlier in 2020.
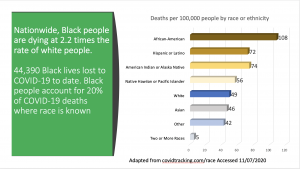
Even more distressing has been the outcry by many American citizens voicing their mistrust of eventual SARS-CoV-2 vaccines that are being rapidly developed and studied as part of Operation Warp-Speed (e.g.- New York Times article– Accessed November 6, 2020). While anti-vaccination sentiment is not uncommon in our country, and around the world, this most recent episode focused on COVID-19 vaccines is acutely grounded in the perception of political interference with the scientific process, as well as remembered abuses of American citizens of color, particularly African-Americans (More than Tuskegee: Understanding Mistrust about Research Participation – J Health Care Poor Underserved. 2010 Aug; 21(3): 879–897). Ultimately, this mistrust may prove to be a losing proposition for African-Americans, Hispanic/ Latinos, and Native American Indians since these communities have mortality rates that are 2.2 times (African-Americans) to 1.5 times ( Hispanic/ Latinos, and Native Indians) that of their Caucasian counterparts (See Image 1). Vaccinations offer an opportunity to defend against the critically deleterious health outcomes that may be associated with natural human infection from SARS-CoV-2. To refuse the SARS-CoV-2 vaccine certainly increases the chances of succumbing to serious long-term health complications, or even death.
Communication from the African-American Scientific Community (Enhanced Transparency)
Recognizing the potentially negative impacts of perceived interference in the scientific process to create these vaccines, multiple national organizations have created ad hoc committees to provide oversight into the clinical research and manufacturing practices of the companies and entities developing the vaccines. The American Medical Association directly engaged the US Food and Drug Administration (FDA) by sending a letter encouraging increased transparency and communication into COVID-19 vaccine development. This engagement seeks to assuage the fears of the public that the SARS-CoV-2 vaccine development is rushed and politicized, which increased the public’s concerns that the vaccines may be unsafe or not effective.
In thorough acknowledgement of the profound historical mistrust from African-American communities with scientific clinical research, the National Medical Association (NMA) formed an interdisciplinary COVID-19 Task Force to directly engage the FDA, as well as the pharmaceutical companies involved in COVID-19 vaccine research and development. The task force includes NMA physicians who serve on the Centers for Disease Control and Prevention (CDC) Advisory Committee on Immunization Practices (ACIP), Infectious Disease Society of America, Pediatric Infectious Disease Society, and the CDC Health Equity Workgroup. Also included are pharmacists, nurses, and epidemiologists all with the goal of reviewing any SARS-CoV-2 vaccine developed will be safe and effective for all American citizens, and particularly those of color, that choose to receive the vaccine.
The goal of the NMA COVID-19 task force will be to provide timely recommendations to physicians and people in communities about any Emergency Use Authorizations (EUA) and eventual approvals by the FDA for COVID-19 vaccines and therapeutics presently being evaluated in clinical trials. Next, effective and accurate communication can be provided directly to communities of color about the safety end effectiveness of these vaccines through their trusted health providers. Hopefully, this will overcome the mistrust that many from the mentioned communities are feeling, and thus give themselves a chance to prevent becoming infected with this terrible virus.
Different Types of Vaccine Mechanisms for Antibody Stimulation
While people in communities may be waiting for “A Vaccine”, actually there are multiple companies developing several types of vaccines with different immunological mechanisms of action. A small number of companies are in Phase II or III in their clinical research process. In actuality, there are over 200+ COVID-19 vaccines in development worldwide. Almost 40 are in some phase of clinical trials. The general perception from the public is that vaccine development just started once the COVID-19 pandemic occurred. In truth, serious coronavirus vaccine development began when the original SARS was prevalent in 2002-04 (Tripp RA, et al. “Monoclonal antibodies to SARS-associated coronavirus (SARS-CoV): identification of neutralizing and antibodies reactive to S, N, M and E viral proteins” Journal of Virological Methods. 128 (1–2): 21–28 , Sept 2005. doi:10.1016/j.) Once this current COVID-19 pandemic began, world-wide researchers had a 15-year history of studying coronavirus vaccine platforms.
For illustrative purposes, I show only four companies with SARS-CoV-2 vaccine clinical trials in the United States (See Image 2); I have no financial relationships with any of the companies shown. People receiving COVID-19 vaccines in the near future must be notified, in lay terms, of the type of vaccine being provided, the company, and generally how it will produce immunity. Two of the vaccines utilize messenger RNA platforms to elicit an antibody-producing immune response in the recipient. This is a new vaccine technology, and can be manufactured relatively quickly when compared to the other vaccines. It is expected that the two companies that produce this version of the SARS-CoV-2 vaccine, Moderna and BioNTech/Pfizer, will file their Phase III results with the FDA first, and likely seek Emergency Use Authorization approval before the end of 2020. To date, the messenger RNA vaccines appear to be safe in clinical trials. The other companies in Image 2 are likely to file for FDA approval in early 2021. It should be noted that of the companies shown in Image 2, only BioNTech/Pfizer did not receive any public money from the US Government to fund their research.
For many of the companies, mass manufacturing of their vaccines began in summer 2020 in order to get an early boost in producing enough doses for large scale distribution once approved. All of the research AND manufacturing processes are certain to be scrutinized by the organizations mentioned earlier (NMA and AMA) for overall safety and effectiveness of the vaccines. These companies have taken the financial risk of their vaccines not enduring the rigor of clinical trials, which could in turn lead to their product not being approved by the FDA.
Equitable Distribution of Manufactured Vaccine Product
On the opposite side of the mistrust of COVID-19 vaccines, there are many health-focused and community organizations that will be monitoring and advocating for the equitable distribution of the vaccines once they have been approved by the FDA. Some lead organizations are the National Academies of Science, Engineering, and Medicine. They formed a group called the Committee on the Equitable Allocation of Vaccine for the Novel Coronavirus, which issued a guidance document that provided a four-phase allocation plan for COVID-19 vaccines. The goal is to account for the most vulnerable and most impacted individuals and communities affected by the pandemic (See Image 3). The earliest vaccine allocations will most likely be provided to front-line healthcare workers, and those individuals at highest risk for severe morbidity and mortality (underlying health conditions (chronic included) and older adults living on congregate living conditions). African-Americans carry a disproportionate share of chronic cardiovascular conditions such as diabetes and hypertension, greatly increasing their mortality potential once infected by SARS-CoV-2. Subsequent allocations should be provided to a larger majority of Americans in numerous components of society (teachers, essential workers, etc), and those with moderate health conditions. The next allocations shall go to young adults, lower transmission-risk employees, and finally all persons residing in the United States (National Academies of Sciences, Engineering, and Medicine 2020. Framework for Equitable Allocation of COVID-19 Vaccine. Washington, DC: The National Academies Press). Again, input and vigilance from organizations such as the National Medical Association and the American Medical Association will be critical to insure that all Americans that choose to be vaccinated will have equitable access.
Taking the Opportunity to Live a Quality Life
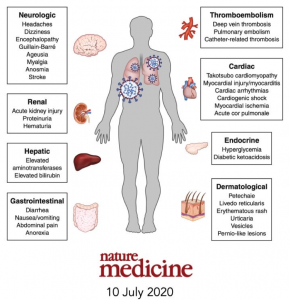
In my previous article “Coronavirus Vaccines – ‘Trust, But Definitely Verify!‘”, the attempt was made to convey that COVID-19 vaccination offers the best opportunity to significantly bend the arc of poor health outcomes caused by SARS-CoV-2 in a more positive direction. The metric of measuring deaths from COVID-19 is not the only concerning statistic, as potentially hundreds of thousands of infected patients may find themselves coping with long-term health challenges well after their infectious state has resolved ( Rubin R. As Their Numbers Grow, COVID-19 “Long Haulers” Stump Experts. JAMA. 2020;324(14):1381–1383). With the known possible negative effects on multiple organ systems in the human body (See Image 4), individuals should make every attempt to protect themselves against this virus through vaccination. Additional and continual communication with the public will certainly be necessary to achieve this public health outcome. In order to achieve this level of trust, the FDA must be overly cooperative and transparent with the NMA, the AMA, and the public during the vaccine approval process once they are adequately proved to be safe and effective for the majority of patients that receive them. Healthcare providers across the country, as they have done and will continue to do throughout the pandemic, stand ready to serve the public health interests to maintain as healthy a society as possible.
Image 1
Image 2
Image 3
Image 4