The global pandemic caused by SARS-CoV-2 (COVID-19) has placed unprecedented demands on United States of America (USA) healthcare systems in 2020. Images of hospitals overrun with COVID-19 patients needing emergency care (often requiring ventilators), shortages of personal protective equipment, overworked healthcare providers (mainly physicians and nurses), and chaotic clinical environments were seen not only in the USA, but worldwide in numerous countries. Countless healthcare providers are often pulled from their normal responsibilities to attend to the overwhelming numbers of patients in the most affected cities and states.
As the COVID-19 pandemic continued to evolve, a call-to-action was issued by the US Department of Health and Human Services, Assistant Secretary for Health, Brett P. Giroir, M.D. His office issued an authorization under the Public Readiness and Emergency Preparedness Act (PREP) allowing licensed pharmacists to order and administer COVID-19 tests that the U.S. Food and Drug Administration (FDA) has authorized. The statement issued by HHS Secretary Alex Azar read: “In an effort to expand testing capabilities, we are authorizing licensed pharmacists to order and administer COVID-19 tests to their patients. The accessibility and distribution of retail and independent community-based pharmacies make pharmacists the first point of contact with a healthcare professional for many Americans. This will further expand testing for Americans, particularly our healthcare workers and first responders who are working around the clock to provide care, compassion and safety to others.”
This PREP Act authorization has been followed by similar state-level executive orders from the governor of Florida, Governor Ron Desantis, and the governor of New York, Governor Andrew Cuomo, among others. This allows pharmacists, especially at the community level, to provide greater accessibility for people to receive COVID-19 testing. It is universally agreed that significant expansion of testing is necessary to assist the identification of potentially infected individuals. Contact tracing can then occur more efficiently, and isolation of infected persons can become more targeted and precise. This also places an additional responsibility upon pharmacists to further engage in the fight against COVID-19 beyond what has already been accomplished.
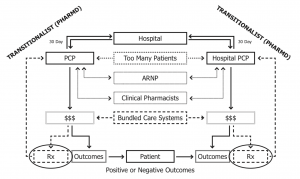
Expanded COVID-19 testing by pharmacists must also achieve proper reporting mechanisms to regional departments of health, as well as other primary care providers if known. This moment also offers an opportunity to establish necessary platforms to accomplish interprofessional interoperability between patients, pharmacists, and primary care providers. Necessary exchanges of pertinent clinical information between health providers is long overdue. A theoretical model of newly emerging roles for pharmacists is shown below (Figure 1):
Figure 1 – original from Kevin B. Sneed, PharmD, FNAP, FNPHA
In previous posts I have fervently advocated for the expanded utilization of mobile health technologies (mHealth) as a means of implementing a new type of data collection for medication therapies. Pharmacists can play a major role in the measurement and monitoring of medications, leading to more complete optimization of medication therapies. In a recent conversation with a physician colleague, I stated that just because a medication was prescribed, and the patient took the medication, does not mean it worked, or achieved a metric-driven outcome. During this COVID-19 pandemic event, numerous reports of patients suffering strokes or myocardial infarctions have been documented. (1Zhang J, Wang X, Jia X, et al. Clin Microbiol Infect. 2020; 2 McMichael TM, Clark S, Pogosjans S, et al. MMWR Morb Mortal Wkly Rep. 2020;69(12):339-342; 3 Roncon L, Zuin M, et al. J Clin Virol. 2020;127:104354.). The increased internal vascular bio-inflammatory status of patients is likely a strong contributor to these events. I would suggest that if mHealth technologies were already in widespread use, concomitant with enhanced implementation of telehealth platforms, the incidence of severe morbidity and mortality of patients with chronic diseases requiring multiple medications may have been fewer in number. Further, facilitated implementation of mHealth and telehealth in underserved/ underrepresented communities may have attenuated the severe negative clinical impact in these populations. (Coronavirus And Health Equity- Again This (Too) Is Predictable – KBSneed)
Expanded COVID-19 testing is necessary to succeed in providing proper public health surveillance of viral spread containment strategies. Community pharmacies are keenly positioned to contribute meaningfully to these community strategies. Issues of PPE for pharmacists and staff, as well as costs of the tests remain key items to be figured out. The enhanced engagement of pharmacists during this COVID-19 pandemic will be essential in providing needed support to already strained health systems. There can be little disagreement that pharmacists should, and must, enhance our engagement in healthcare activities beyond purveyors of medicinal product and join the other health care clinicians in the war against COVID-19. The public health, societal, and economic implications are too great for anyone not to engage.