University of South Florida study suggests a new approach to inhibit the buildup of brain-damaging tau tangles associated with FTLD, Alzheimer’s disease and related dementias
TAMPA, Fla. (Feb. 18, 2020) — The protein β-arrestin2 increases the accumulation of neurotoxic tau tangles, a cause of several forms of dementia, by interfering with removal of excess tau from the brain, a new study by the University of South Florida Health (USF Health) Morsani College of Medicine found.

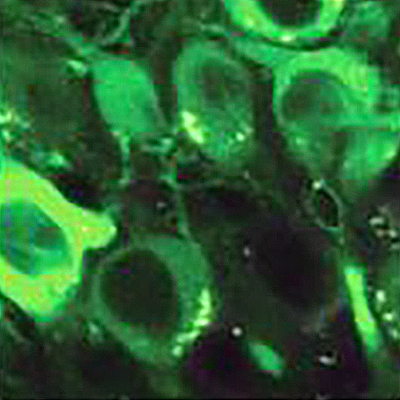
A beta-arrestin2 oligomer (foreground) shown within a nerve cell (background). Oligomerized beta-arrestin2 plays a central role in impairing tau clearance and the development of tau aggregates (magenta) in frontotemporal lobe degeneration and Alzheimer’s disease. | Image courtesy of artist Cynthia Greco and Eric Lewandowski (beta-arrestin2 protein modeling)
The USF Health researchers discovered that a form of the protein comprised of multiple β-arrestin2 molecules, known as oligomerized β-arrestin2, disrupts the protective clearance process normally ridding cells of malformed proteins like disease-causing tau. Monomeric β-arrestin2, the protein’s single-molecule form, does not impair this cellular toxic waste disposal process known as autophagy.
Their findings were first published Feb. 18 in the Proceedings of the National Academy of Science (PNAS).
The study focused on frontotemporal lobar degeneration (FTLD), also called frontotemporal dementia — second only to Alzheimer’s disease as the leading cause of dementia. This aggressive, typically earlier onset dementia (ages 45-65) is characterized by atrophy of the front or side regions of the brain, or both. Like Alzheimer’s disease, FTLD displays an accumulation of tau, and has no specific treatment or cure.
“Our research could lead to a new strategy to block tau pathology in FTLD, Alzheimer’s disease and other related dementias, which ultimately destroys cognitive abilities such as reasoning, behavior, language, and memory,” said the paper’s lead author JungA (Alexa) Woo, PhD, an assistant professor of molecular pharmacology and physiology and an investigator at the USF Health Byrd Alzheimer’s Center.
“It has always been puzzling why the brain cannot clear accumulating tau” said Stephen B. Liggett, MD, senior author and professor of medicine and medical engineering at the USF Health Morsani College of Medicine. “It appears that an ‘incidental interaction’ between β-arrestin2 and the tau clearance mechanism occurs, leading to these dementias. β-arrestin2 itself is not harmful, but this unanticipated interplay appears to be the basis for this mystery.”

The USF Health research team included, from left: Stephen Liggett, MD, senior author; David Kang, PhD, coauthor; and JungA (Alexa) Woo, PhD, lead author. | Photo by Freddie Coleman
“This study identifies beta-arrestin2 as a key culprit in the progressive accumulation of tau in brains of dementia patients,” said coauthor David Kang, PhD, professor of molecular medicine and director of basic research for the Byrd Alzheimer’s Center. “It also clearly illustrates an innovative proof-of-concept strategy to therapeutically reduce pathological tau by specifically targeting beta-arrestin oligomerization.”
The two primary hallmarks of Alzheimer’s disease are clumps of sticky amyloid-beta (Aβ) protein fragments known as amyloid plaques and neuron-choking tangles of a protein called tau. Abnormal accumulations of both proteins are needed to drive the death of brain cells, or neurons, in Alzheimer’s, although the tau accumulations now appear to correlate better with cognitive dysfunction than Aβ, and drugs targeting Ab have been disappointing as a treatment. Aβ aggregation is absent in the FTLD brain, where the key feature of neurodegeneration appears to be excessive tau accumulation, known as tauopathy. The resulting neurofibrillary tangles — twisted fibers laden with tau — destroy synaptic communication between neurons, eventually killing the brain cells.
“Studying FTLD gave us that window to study a key feature of both types of dementias, without the confusion of any Aβ component,” Dr. Woo said.
Monomeric β-arrestin2 is mostly known for its ability to regulate receptors, molecules on the cell that are responsible for hormone and neurotransmitter signaling. β-arrestin2 can also form multiple interconnecting units, called oligomers. The function of β-arrestin2 oligomers is not well understood.
The monomeric form was the basis for the laboratory’s initial studies examining tau and its relationship with neurotransmission and receptors, “but we soon became transfixed on these oligomers of β-arrestin2,” Dr Woo said.

Neurofibrillary tangles laden with tau (stained red) destroy synaptic communication between neurons, eventually killing the brain cells. This tau pathology is a feature of frontotemporal dementia, Alzheimer’s disease and several other dementias. | Image courtesy of David Kang
Among the researchers’ findings reported in PNAS:
– Both in cells and in mice with elevated tau, β-arrestin2 levels are increased. Furthermore, when β-arrestin 2 is overexpressed, tau levels increase, suggesting a maladaptive feedback cycle that exacerbates disease-causing tau.
– Genetically reducing β-arrestin2 lessens tauopathy, synaptic dysfunction, and the loss of nerve cells and their connections in the brain. For this experiment researchers crossed a mouse model of early tauopathy with genetically modified mice in which the β–arrestin2 gene was inactivated, or knocked out.
– Oligomerized β-arrestin2 — but not the protein’s monomeric form – increases tau. The researchers blocked β-arrestin-2 molecules from binding together to create oligeromized forms of the protein. They demonstrated that pathogenic tau significantly decreased when β-arrestin2 oligomers are converted to monomers
– Oligomerized β-arrestin2 increases tau by impeding the ability of cargo protein p62 to help selectively degrade excess tau in the brain. In essence, this reduces the efficiency of the autophagy process needed to clear toxic tau, so tau “clogs up” the neurons.
– Blocking of β-arrestin2 oligomerization suppresses disease-causing tau in a mouse model that develops human tauopathy with signs of dementia.
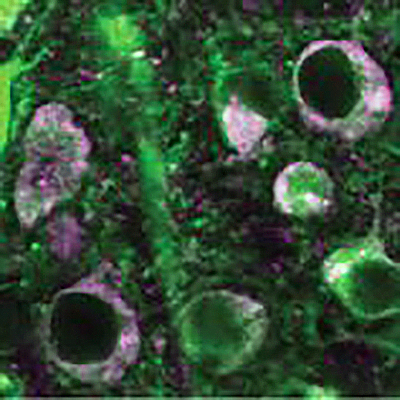
Above: Control nerve cells (green), in which oligomerized beta-arrestin-2 contributes to the accumulation of disease-causing tau (magenta). Below: When the neurons are transduced with b-arrestin2 oligomerization blocking viruses, tau pathology is dramatically reduced. | Images appearing in PNAS (Fig 6D) courtesy of Alexa Woo
“We also noted that decreasing β-arrestin2 by gene therapy had no apparent side effects, but such a reduction was enough to open the tau clearance mechanism to full throttle, erasing the tau tangles like an eraser,” Dr. Liggett said. “This is something the field has been looking for — an intervention that does no harm and reverses the disease.”
“Based on our findings, the effects of inhibiting β-arrestin2 oligomerization would be expected to not only inhibit the development of new tau tangles, but also to clear existing tau accumulations due to the mechanism of enhancing tau clearance,” the paper’s authors conclude.
The work is consistent with a new treatment strategy that could be preventive for those at risk or with mild cognitive impairment, and also for those with overt dementias caused by tau, by decreasing the existing tau tangles.
The study was supported in part by grants from the National Institutes of Health, National Institute on Aging.